Study population
For the current project, we used a cohort of 38 donor–recipients pairs from the REnal Protection Against Ischaemia–Reperfusion in transplantation (REPAIR) study. Briefly, in the original study, patients took part in a multicenter double-blind randomized clinical trial (ISRCTN registry number 30083294) which investigated whether remote ischaemic preconditioning (RIPC) improved renal function after living-donor kidney transplantation. Full details of the REPAIR study have been described previously15,16. In brief, between 4 January 2010 and 29 April 2013, 406 live donor–recipient pairs aged ≥ 18 years were included from thirteen tertiary care hospitals in the UK, the Netherlands, Belgium and France. Patients who used medicines that modulate preconditioning pathways (ATP-sensitive potassium channel opening or blocking drugs, or ciclosporine), had iodine sensitivity (contraindicating iohexol), or required antibody removal (ABO- or HLA-incompatible transplants) were excluded. Each donor–recipient pair was randomized to one of four groups: sham RIPC, early RIPC (immediately before surgery), late RIPC (24 h before surgery) and dual RIPC (early and late RIPC). Both donor and recipient received the same intervention (active RIPC or sham RIPC) at the two time points. Patients were followed up to 5 years after transplantation. The REPAIR study showed a sustained improvement in eGFR after early RIPC, compared with control from 3 months to 5 years after living kidney transplantation (adjusted mean difference: 4.71 mL/min/1.73 m2 [95% confidence interval, CI 1.54–7.89]; P = 0.004)16. The REPAIR study was conducted in accordance with the Declaration of Helsinki and approved by the Joint University College London (UCL)/University College London Hospital (UCLH) Committees on the Ethics of Human Research in June 2009 (reference number 09/H0715/48). Local research ethics committee approvals were gained at all recruiting sites and hospitals involved. Written informed consent was obtained from all patients15.
Urine samples and measurements
For the present study, we used heparinised plasma and spot urine samples from 38 out of the original 55 Dutch REPAIR donor–recipient pairs, for whom all samples were available. Donor plasma and urine samples were collected twice, namely 24 h before and 24 h after nephrectomy. Recipient plasma and urine samples were only collected 24 h after kidney transplantation, resulting in a total amount of 112 (= 3 × 38 minus 2 missing) plasma and 114 (= 3 × 38) urine samples. All blood and urine samples were centrifuged at 400g for 10 min and the supernatant was aspirated, partitioned into 2 mL aliquots and stored at − 70 °C to − 80 °C. Samples underwent one freeze–thaw cycle and were stored for a maximum of 10 years prior to chemical analysis.
All plasma and urinary measurements were performed from September 2020 to February 2021. Plasma creatinine, cystatin C, albumin, urea, B2M and urinary creatinine, albumin and B2M were measured using a Cobas c502 analyzer (Roche Diagnostics, Mannheim, DE) according to the manufacturer’s instructions. GFR was estimated based on plasma creatinine (eGFRcr), plasma cystatin C (eGFRcysC) and combined plasma creatinine–cystatin C (eGFRcr–cysC), using the Chronic Kidney Disease Epidemiology Collaboration equations from 2012, taking into account age, sex and race17. For long-term eGFR at 3 and 12 months after transplantation only eGFRcr was available since these data were extracted from patient files. Urinary osmolality was measured using Osmo-Station OM-6060, ARKRAY Inc., Kyoto, Japan. Liquicheck (BIO-RAD, Irvine, CA) quantitative urine controls were used for internal QC, mean (SD, %CV) values were 310 mOsmol/kg (2 mOsmol/kg, 0.6%, n = 29) and 551 mOsmol/kg (3 mOsmol/kg, 0.6%, n = 29) for normal and abnormal quality control.
Concentrations of urinary TIMP-2, IGFBP7, KIM-1 and NGAL were measured using sandwich enzyme-linked immunosorbent assays (ELISA) according to manufacturer’s instructions (Cat. Nr. DTM200, R&D systems, Minneapolis, MN for TIMP-2, Cat. Nr. EK0991, Boster Biological Technology, Pleasanton, CA for IGFBP7, Cat. Nr. DY1750, R&D systems, Minneapolis, MN for KIM-1 and Cat. Nr. DY1757, R&D systems, Minneapolis, MN for NGAL). Sample dilutions used were checked to be within the linear range, and the same lot numbers (P238376 for TIMP-2, 6371674817 for IGFBP7) were used throughout the entire study. For TIMP-2 and IGFBP7, two internal low and high-level quality control (QC) samples (QC1 and QC2), consisting of pooled urine, were analysed in triplicate on each sample plate to assess the stability of the assay. For TIMP-2, total analytical imprecision, expressed as coefficient of variation % (CV%), was 3.6% at mean (SD) 172 (6) pmol/L for QC1 (n = 15) and 4.2% at 243 (10) pmol/L for QC2 (n = 15). For IGFBP7 CV% was 9.4% at 757 (71) pmol/L for QC1 (n = 20) and 10.9% at 2053 (224) pmol/L for QC2 (n = 20).
Additional data
Baseline characteristics 24 h before kidney transplantation and data on laboratory measurements during follow-up were provided from the original REPAIR study database. Participant characteristics included age, sex, ethnicity, RIPC randomised group, BMI, systolic blood pressure, primary kidney disease and whether there was a history of dialysis or a previous kidney transplantation.
Statistical analysis
Baseline characteristics of the living kidney transplant donors and recipients are presented as mean with standard deviation (SD) or median with interquartile range (IQR) for continuous data when appropriate. Categorical variables are expressed as proportions. First, in 38 donors 24 h before and after nephrectomy and 38 donor–recipient pairs 24 h after transplantation, mean (SD) and mean differences (95% CI) of plasma creatinine, cystatin C, eGFRcr, eGFRcys, eGFRcr-cys and B2M and median concentrations (IQR) and differences (95% CI) of urinary albumin-to-creatinine ratio (ACR) were calculated. Mean differences were calculated using the paired t test and median differences using the Wilcoxon signed rank test.
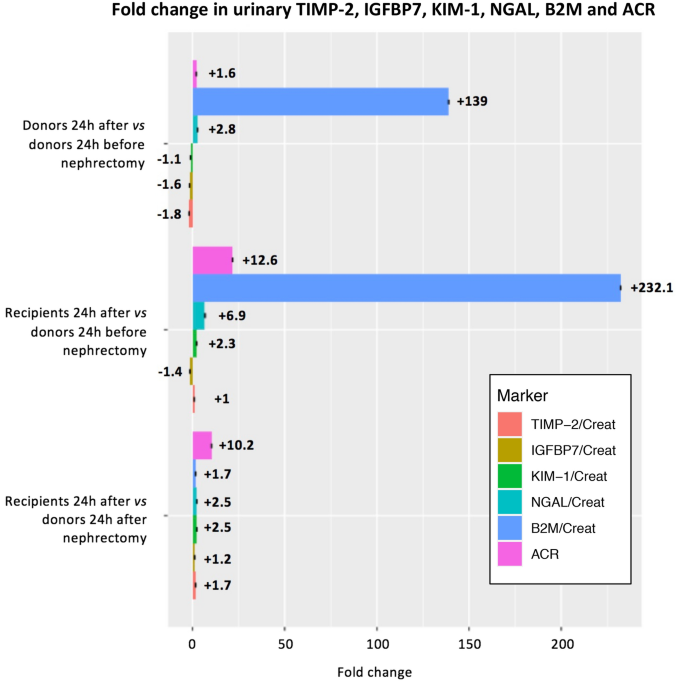
Second, median (IQR) concentrations and median differences (95% CI) of urinary B2M, TIMP-2, IGFBP7, KIM-1 and NGAL standardized for urinary creatinine were calculated for the 38 living donor (24 h before and after nephrectomy) and recipient (24 h after surgery) pairs. Results were also visualized with boxplots. Third, we calculated changes between biomarkers measured in donors and recipients 1 day after transplantation, compared to biomarkers measured in donors 1 day before and after transplantation. Fourth, associations between urinary levels of B2M, TIMP-2, IGFBP7, KIM-1 and NGAL and concurrent eGFRcr-cys were assessed using linear regression analysis, both crude and adjusted for donor age and sex, in the 38 living donor (24 h before and after surgery) and recipient (24 h after surgery) pairs. Additionally, using similar models, the associations between change in urinary levels of B2M, TIMP-2, IGFBP7, KIM-1 and NGAL and change in eGFRcr-cys from 24 h before to 24 h after nephrectomy and eGFR cr-cys at 1 year after nephrectomy were assessed in the 38 living donors. In all regression analyses, B2M, TIMP-2, IGFBP7, KIM-1 and NGAL were divided by their SD or log-transformed by the natural logarithm to normalize their distributions.
In all analyses apart from those investigating long-term eGFR, concentrations of urinary B2M, TIMP-2, IGFBP7, KIM-1 and NGAL were corrected for urinary creatinine and osmolality separately and expressed as a ratio of the biomarker divided by creatinine. All analyses were performed using R version 3.5.1 (R Core Team, Vienna, Austria).
- The Renal Warrior Project. Join Now
- Source: https://www.nature.com/articles/s41598-024-62246-1
