Antibodies
Antibodies against OTUD5 (#21002-1-AP), FLAG-Tag (#20543-1-AP), HA-Tag (#51064-2-AP), His-Tag (#66005-1-IG), Rabbit IgG (#30000-0-AP), Mouse IgG (#B900620) were purchased from Proteintech (Wuhan, China). Antibodies against ERK (#sc-514302), P-ERK (#sc-7383), JNK (#sc-7345), and P-JNK (#sc-6254) were purchased from Santa Cruz (CA, USA). Antibodies against TAK1 (#5206S), P-TAK1 (Ser412) (#9339S), TAB2 (#3745S), Ubiquitin (P4D1) (#3936), P38 (#8690S) and P-P38 (#4631S) were purchased from Cell Signaling Technology (MA, USA). Antibodies against GAPDH (#MB001) were purchased from Bioworld (Missouri, USA).
Animal experiments
All animal care and handling procedures were conducted according to the National Institutes of Health (USA) guidelines and approved by the Wenzhou Medical University Animal Policy and Welfare Committee (Approval number: Wydw2021-0182). Podocyte-specific Otud5 knockout Mice (Nphs1-Cre OTUD5fl/fl mice; OTUD5CKO) were obtained from Gempharmatech Co., Ltd (Jiangsu, China). The genotype of OTUD5CKO was maintained by crossing the C57BL/6JGpt-Otud5em1Cflox/Gpt mouse (OTUD5fl/fl, strain No. T052115) and the C57BL/6JGpt-H11em1Cin(Nphs1-iCre)/Gpt mouse (Myh6-iCre, strain No. T005680). The mice were housed in an environmentally controlled room at 22 ± 2.0 °C and 50% ± 5% humidity, with a 12-hour light/dark cycle, and were fed standard rodent chow and tap water. All animal experiments were conducted in a blinded manner.
-
(1)
Streptozotocin (STZ)-induced type 1 diabetic kidney disease in mice. Six-week-old male OTUD5CKO mice and their littermate OTUD5fl/fl mice were randomly divided into 4 groups: OTUD5fl/fl + sham group, OTUD5fl/fl + STZ group, OTUD5CKO + sham group, OTUD5CKO + STZ group (n = 6). STZ was purchased from Sigma-Aldrich (S0130, MO, USA). Low-dose STZ (50 mg/kg) dissolved in 0.1 M sodium citrate buffer was intraperitoneally injected for 5 continuous days, while an equal amount of sodium citrate buffer (pH 4.5) was used as the control. Blood glucose (BG) levels were measured and recorded using a glucometer (One Touch Ultra Easy, Life Scan, USA), with fasting blood glucose (FBG) levels above 16.7 mmol/L considered indicative of diabetes. All mice were given free access to food and water for 16 weeks.
-
(2)
High-fat diet feeding plus STZ injection (HFD/STZ) induced type 2 diabetic kidney disease in mice. Six-week-old male OTUD5CKO mice and their littermate OTUD5fl/fl mice were randomly divided into 4 groups: OTUD5fl/fl + sham group, OTUD5fl/fl + HFD/STZ group, OTUD5CKO + sham group, and OTUD5CKO + HFD/STZ group (n = 6). The high-fat diet (HFD, 60 kcal% from fat) was purchased from Research Diets (D12492, USA). A single dose of STZ (100 mg/kg) was injected after 4 weeks of feeding the HFD or a control diet. The mice continued to be maintained on an HFD or control diet for another 12 weeks.
-
(3)
Pharmacological inhibition of TAK1 in type 2 diabetic mice. Six-week-old male OTUD5CKO mice and their littermate OTUD5fl/fl mice were randomly divided into 4 groups: OTUD5fl/fl + T2DM group, OTUD5fl/fl + T2DM + Takinib group, OTUD5CKO + T2DM group, and OTUD5CKO + T2DM + Takinib group (n = 6). The TAK1 inhibitor, Takinib, was purchased from MedChemExpress (HY-103490, California, USA). Takinib (50 mg/kg) was dissolved in Sodium carboxymethyl cellulose (CMC-Na) and administered orally every other day.
-
(4)
Podocyte-specific over-expression of OTUD5 in type 2 diabetic mice. The recombinant adeno-associated virus serotype 9 (AAV9) carrying OTUD5 or EV cDNA with a podocyte-specific promoter NPHS1 (Bioqure Genetech, Hangzhou, China) was used for over-expression of OTUD5 or control. Six-week-old male OTUD5fl/fl mice were randomly divided into 2 groups: OTUD5fl/fl + T2DM + AAV-EV group and OTUD5fl/fl + T2DM + AAV- OTUD5 group (n = 6). AAV9 was administered via the tail vein at a dose of 2E + 11 vg.
Before sacrifice, mice were euthanized under anesthesia using pentobarbital sodium (50 mg/kg, intraperitoneally). Blood samples and 24-hour urine samples were collected for biochemical analysis. Kidney tissues were harvested for histopathological analysis. Protein or RNA was extracted from kidney tissue for subsequent analysis.
Cell culture and transfection
The mice renal podocyte cell line (MPC5) was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). MPC5 cells were cultured in RPMI 1640, 10% fetal bovine serum (Meilunbio, PWL001) and 10 IU/mL of recombinant murine c-interferon (IFN-γ, Invitrogen, CA, USA) at 33 °C and 5% CO2. To induce differentiation, the cells were transferred to 37 °C for 10–14 days and the medium was replaced without IFN-γ. The NIH/3T3 cell line was purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China) and cultured in DMEM containing 4.5 g/L glucose. The culture medium was supplemented with 10% fetal bovine serum (Meilunbio, PWL001), 100 U/mL penicillin, and streptomycin (Sbjbio, Nan Jing, BC-CE-007) at 37 °C with 5% CO2.
Primary podocytes were extracted as described below. The kidney cortex was finely chopped into 1 mm3 pieces and then digested in collagenase IV buffer (C8160, Solarbio, Beijing, China) at 37 °C for 15 minutes. The digested tissue was subsequently passed through cell filters of decreasing sizes: 150 μm, 75 μm, and finally 40 μm. After a precipitation period of 1–2 minutes, large debris tubules adhered to the bottom of the petri dish, while most of the glomeruli floated and small debris tubules were present in the supernatant. The supernatant was then passed through the 40 μm filter again, repeating this step until a highly purified glomerular supernatant was obtained. After 7 days, the glomerular cells were digested and then filtered with a 40 µm cell filter. Extracted primary podocytes were further incubated.
Gene expression was silenced using small interfering RNA (siRNA) purchased from GenePharma (Shanghai, China). MPC5 cells were transfected with siRNA targeting OTUD5 (sense: GGACAUGCAUGAGGUUGUUTT, anti-sense: AACAACCUCAUGCAUGUCCTT) with LipofectAMINE™ 2000 (#L3000015, Thermo Fisher Scientific, German).
The overexpression plasmids (Flag-OTUD5-WT, Flag-OTUD5-C224A, His-TAK1-WT, His-TAK1-K34R, His-TAK1-K158R, His-TAK1-K209R, and His-TAK1-K562R) were obtained from Tsingke Biotechnology Co., Ltd. (Beijing, China). Plasmids (HA-Ub, HA-K48 Ub, and HA-K63 Ub) were obtained from Addgene. The above plasmids were transfected by LipofectAMINE™3000 (L3000150, Thermo Fisher Scientific, German).
Biochemical analysis of serum and urine samples
Serum creatinine, urea nitrogen, and urine creatinine levels were measured by enzymatic assays using an auto-chemistry analyzer. Urine albumin was detected using an ELISA kit (H127-1-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Histological analysis
Mice kidney tissues were fixed with 4% paraformaldehyde and embedded in paraffin. Hematoxylin and eosin (H&E, G1120, Solarbio, Beijing, China) staining of tissue sections (5 μm) were used to evaluate the histopathological damage. Periodic acid-Schiff (PAS, G1285, Solarbio, Beijing, China) staining was used to evaluate the mesangial dilation in the glomeruli. Images were taken with a light microscope (Nikon, Tokyo, Japan).
Transmission Electron Microscopy
Electron microscope samples were treated and observed by the electron microscope Laboratory of Wenzhou Medical University. The renal cortex was cut into pieces (<1 mm2) and fixed with 2.5% glutaraldehyde at 4 °C. The tissue slices were washed in 0.2 mol/L PBS solution and then incubated with 1% osmic acid at room temperature for 2 hours. After a gradient dehydration process using ethanol, the sections were embedded in Pon812 resin with acetone as solvent at 37 °C overnight. Ultrathin sections were stained with a solution of 2% uranyl acetate and lead citrate and examined under a Philips CM 120 electron microscope (Philips Medical Systems, Inc.). The thickness of the GBM and the number of foot processes were analyzed using NIH ImageJ (National Institutes of Health, Bethesda, MD).
Transcriptome sequencing
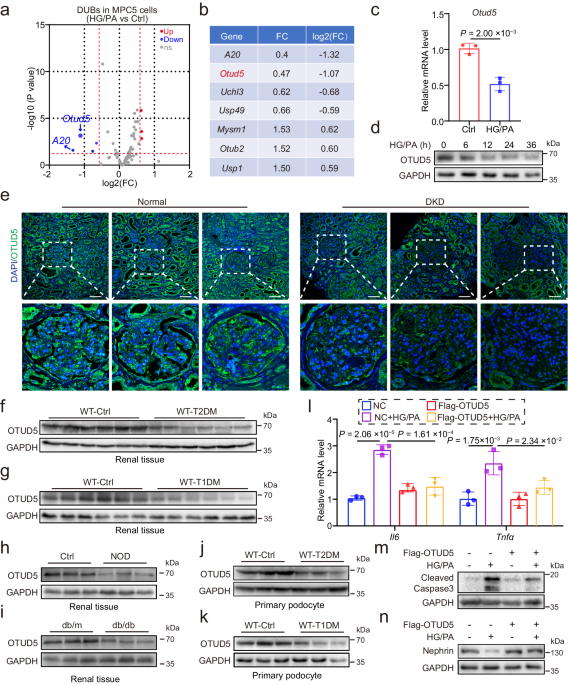
Total RNA from untreated or HG/PA-treated MPC5 was collected using RNAiso Plus and subjected to genome-wide transcriptomic analysis by LC-Bio (Hangzhou, China). The differentially expressed genes (DEGs) were selected with a fold change >1.5 or fold change <0.667. GSEA (https://www.gsea-msigdb.org/gsea/ index.jsp) of the signaling pathways was performed as described by LC-Bio (https://www.lc-bio.cn/).
Western blot
Cultured cells and renal tissues were lysed in RIPA buffer (P0013C, Beyotime Biological, Shanghai, China) containing proteinase inhibitor PMSF (ST506, Beyotime Biological, Shanghai, China). Equal amounts of protein were then separated by SDS-PAGE and transferred to PVDF membranes. The membranes were subsequently blocked in 5% Bovine Serum Albumin (BSA, A8020, Solarbio, Beijing, China) and incubated with primary antibodies overnight at 4 °C. After incubating with a secondary antibody (Beyotime Biological, Shanghai, China) for 1 hour, the membranes were detected by an enhanced chemiluminescence (ECL) system. The quantification of images was analyzed by ImageJ software.
Co-immunoprecipitation (co-IP)
The cell and renal tissue lysates were incubated overnight with specific primary antibodies or control IgG. Protein A + G Agarose (P2012, Beyotime Biological, Shanghai, China) was added to the lysates and incubated at 4 °C for 2 hours to capture the immune complex. The supernatant was then discarded after centrifugation, and the precipitate was washed 5 times with PBS. The protein was eluted with an SDS loading buffer for western blotting.
LC-MS/MS analysis
MPC5 cells were transfected with Flag-vector or Flag-OTUD5 plasmids. Anti-Flag and protein A + G Agarose beads were added to the cell samples. LC-MS/MS analyses were performed on a Q Exactive HF mass spectrometer that was coupled to Easy nLC (Thermo Scientific). Peptide was first loaded onto a trap column (100 μm*20 mm, 5 μm, C18) with 0.1% formic acid, then separated by an analytical column (75 μm*100 mm, 3 μm, C18)) with a binary gradient of buffer A (0.1% Formic acid) and buffer B (84% acetonitrile and 0.1% Formic acid) at a flow rate of 300 nL/min over 60 min. MS data was acquired using a data-dependent top20 method dynamically choosing the most abundant precursor ions from the survey scan (350–1800 m/z) for HCD fragmentation. The MS data were analyzed using MaxQuant software version 1.5.8.3. MS data were searched against the UniProtKB Human database (157,600 total entries, downloaded 07/2017). The trypsin was selected as a digestion enzyme. The maximal two missed cleavage sites and the mass tolerance of 4.5 ppm for precursor ions and 20 ppm for fragment ions were defined for database search. Carbamidomethylation of cysteines was defined as fixed modification, while acetylation of protein N-terminal and Lysine, and oxidation of Methionine, were set as variable modifications for database searching. The database search results were filtered and exported with <1% false discovery rate (FDR) at peptide level and protein level, respectively.
RNA extraction and real‐time quantitative PCR
Total RNA was isolated from cells or renal tissues using TRIzol (#9109, Takara, Japan), and 1ug total RNA was reverse transcribed by HiScript®III All-in-one RT SuperMix reagent (R333-01, Vazyme, Nanjing, China). The quantitative PCR was performed with SYBR Green Master Mix (Q711-02, Vazyme, Nanjing, China) in a Real-Time PCR System (QuantStudioTM 3, Thermo Fisher Scientific, CA, USA). Gene levels were normalized to β-actin, and the relative gene expression was analyzed using the ΔΔ-Ct algorithm. The primers used are listed in Supplementary Table 1.
Human renal biopsy samples
Renal biopsies from 3 diabetic patients and 3 nondiabetic patients were collected from the Department of Pathology, the First Affiliated Hospital of Wenzhou Medical University. The non-diabetic samples were obtained from the healthy kidney poles of individuals who underwent tumor nephrectomies without diabetes or renal disease. All experiments involving human samples were approved by the Ethics Committee in Clinical Research of the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China; Approval number: 2023-0115), and informed consent was obtained from the patients. All the human study participants agreed to participate for free. All aspects of the study followed the Declaration of Helsinki of 1975, revised in 2008. The clinical characteristics of the patients are shown in Supplementary Table 2.
Data and statistical analysis
All data were analyzed using GraphPad Prism 8.0 software (San Diego, CA, USA). Data are expressed as mean ± SD. The unpaired t-test was used for comparisons between two groups and one-way ANOVA analysis (Bonferroni’s correction) was used for comparing multiple groups. P values less than 0.05 were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
- The Renal Warrior Project. Join Now
- Source: https://www.nature.com/articles/s41467-024-49854-1
