Study setting
We conducted a prospective, single-center, open-label, randomized controlled trial that assessed BIA-and clinical criteria-guided volume control using CRRT in patients with sepsis-associated AKI in the ICU of a large tertiary care hospital (Severance Hospital, Seoul, Republic of Korea) (Trial registration: ClinicalTrials.gov, NCT02384525, Registered 10 March 2015, https://clinicaltrials.gov/study/NCT02384525). This study was conducted between June 2017 and October 2021. This study was approved by the institutional review board of Severance Hospital and conducted in accordance with the provisions of the Declaration of Helsinki (institutional review board approval number:4-2014-0791). All participants and/or substitute decision-makers were informed of the study and provided written informed consent.
Participants selection
Participants were eligible for enrollment if they were aged 19 years or older, admitted to the ICU with AKI due to sepsis, and required CRRT. Each case of sepsis was defined according to the consensus conference criteria suggested by the Society of Critical Care Medicine and American College of Chest Physicians36. Briefly, if a patient had a suspected infection and met systemic inflammatory response syndrome criteria (2 or more of following; body temperature < 36 °C or > 38 °C, heart rate > 90 beats/min, respiratory rate > 20 breaths/min or PaCO2 < 32 mmHg, or white blood cell count < 4.0 × 103/μL or > 12.0 × 103/μL) in two consecutive measurements, we diagnosed sepsis. Infection was diagnosed if the causative organisms were isolated by culture studies or were clinically suspected as follows: (1) white blood cells in a normally sterile fluid; (2) perforated viscus; or (3) obvious evidence of infection from imaging tests, including pneumonia and abscess. We included patients with AKI at a level greater than the ‘injury’ stage according to the RIFLE criteria, which was consistent with a more than twofold increase in serum creatinine level compared with baseline or urine output < 0.5 mL/kg/h over 12 h. The exclusion criteria were as follows: (1) patients older than 80 years; (2) patients already receiving kidney replacement therapy due to kidney failure; (3) life expectancy of less than 3 months due to terminal cancer; (4) presence of an intracardiac device, including a pacemaker, implantable cardioverter defibrillator, or cardiac resynchronization therapy; (5) pregnancy or lactation; or (6) generalized exfoliative skin disease. Participants were excluded from the final analysis if their severe hypophosphatemia (serum phosphorus < 2.5 mg/dL) or hypokalemia (serum potassium < 3.5 mg/dL) was not corrected within 12 h after the first detection.
Data collection and measurements
The baseline demographic, clinical, and biochemical characteristics were collected at the time of randomization. Disease severity was determined using the Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores. We assessed vital signs, doses of vasoactive/vasopressor agents, and laboratory test results, including complete blood count, chemistry, blood gas analyses, and lactic acid levels. The dose of inotropic agents was expressed as the vasoactive-inotropic score (VIS) and vasopressor dependency index (VDI), which is a dimensionless variable calculated using the following formulas37,38,39.
$$begin{aligned} VIS & = dopamin ;dose left( {mu g/kg/min } right) + dobutamin e ;dose left( {mu g/kg/min } right) + 100; & & quad times epinephrine; dose left( {mu g/kg/min } right) + 10 times min irone; dose left( {mu g/kg/min } right) & quad + 10000 times vasopressin ; dose left( {units/kg/min } right) + 100 & quad times norepinephrine; dose left( {mu g/kg/min } right) end{aligned}$$
$$VDI = frac{VIS}{{Mean;arterial;pressure left( {mmHg} right)}}$$
Serum creatinine levels were measured using an isotope-dilution mass spectrometry-tractable method, and the estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation40.
BIA measurement
The amount of fluid overload was assessed by BIA using a Body Composition Monitor (BCM) (Fresenius Medical Care, Bad Homburg vor der Hohe, Germany) according to the manufacturer’s instructions within 6 h of CRRT initiation. Briefly, the participants were removed from the metallic devices or accessories. The BCM electrodes were then placed on the dorsum of the hand and foot at the metacarpal and metatarsal sites, respectively. The parameter overhydration was adopted for the amount of fluid overload.
Treatment assignments
CRRT was initiated at the discretion of a consulting nephrologist without considering the patient’s eligibility for this study. Generally, CRRT is prescribed in patients with AKI at a stage greater than the injury stage classified by the RIFLE criteria, with the presence of significant volume overload, uncontrolled hyperkalemia (potassium > 6.5 mEq/L), or severe acidemia (pH < 7.2). CRRT was delivered by Prisma or Prisma Flex machines (Baxter, Deerfield, IL, USA) using ST100 (surface area, 1.0 m2) filter sets. Vascular access for CRRT was obtained by inserting a 14F double-lumen catheter into the internal jugular or femoral vein. The effluent volume was set to achieve clearance rate of 40 mL/kg/h for guaranteeing minimal clearance rate of 35 mL/kg/h considering interruption of CRRT due to special examinations (e.g. computed tomography) or unexpected causes (e.g. machine error)22. All patients received CRRT in the continuous veno-venous hemodiafiltration mode. The replacement and dialysate volumes were set using a 1:1 balanced predilution method22. In the conventional volume control group, volume control was performed by an experienced nephrologist based on clinical parameters such as daily fluid balance, physical examination, and review of chest radiographs. In the BIA-guided volume control group, the amount of daily volume reduction was determined to be one-third of overhydration, and the strategy of volume control was maintained for 72 h (Supplementary Fig. S3). After 72 h of randomization, volume control in both groups was performed at the discretion of an experienced nephrologist.
Patients remained on CRRT until kidney function recovered, transfer to conventional hemodialysis, withdrawal of CRRT as part of life support, or death. The decision to wean a patient from CRRT was made by a nephrologist when the patient recovered hemodynamic stability during intermittent hemodialysis or had considerable urine output.
Patients eligible for enrollment were informed of the study and those who provided written consent were randomly assigned in a 1:1 ratio to each treatment group using a centralized computer-generated adaptive randomization scheme by a neutral person who was not involved in the trial at the time of CRRT initiation.
Sample size estimation and study population
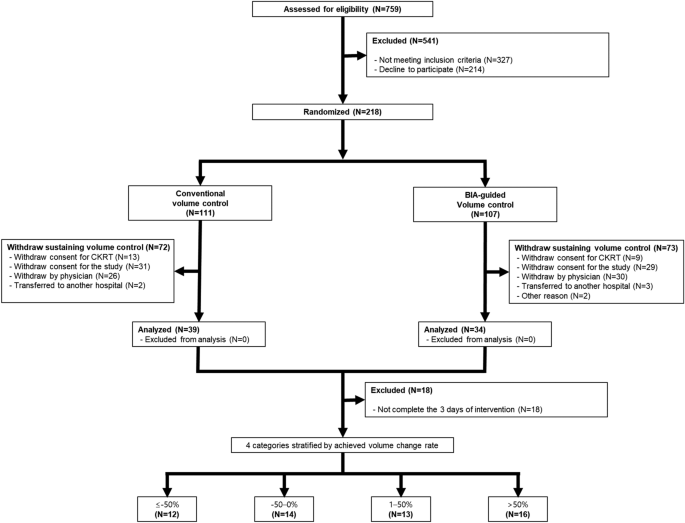
The sample size was estimated based on the following assumptions. The mortality rate in the control group was estimated to be 60% based on previous studies22,41,42. This study aimed to demonstrate a ≥ 20% reduction in mortality rate. To detect a significant difference in the primary outcome with a two-sided type I error of 0.05 and 80% power, we estimated that 82 participants would be required for each arm. We aimed to enroll at least 109 patients to allow a dropout rate of 25% in individual treatment groups. A total of 73 patients were included in the primary analysis (intention-to-treat analysis) (Fig. 2). In the secondary analysis to investigate the association between the achieved volume accumulation rate and clinical outcomes, we excluded 18 patients who did not complete the 3 days of intervention. Finally, 55 participants were included in the secondary analysis (Fig. 2).
Flow diagram of study participants. From June 2017 to October 2021, a total of 759 patients who initiated continuous renal replacement therapy at Severance Hospital (Seoul, Republic of Korea) were initially assessed for eligibility. According to inclusion and exclusion criteria, 73 patients were included in the primary analysis (intention-to-treat analysis). After excluding 18 patients who did not complete the 3 days of intervention, 55 subjects were included in the secondary analysis. BIA bioelectrical impedance analysis; CRRT continuous renal replacement therapy.
End points
The outcome of interest was death from any cause within 28 and 90 days of randomization. ICU and in-hospital deaths were also evaluated. ICU death and in-hospital death were defined as death during the ICU stay or hospitalization, regardless of the time point. Additionally, we set death from any cause within 7 days of randomization as a short-term outcome.
Statistical analysis
The data were analyzed using Stata 15.1 (Stata Corporation). All data are expressed as the mean (standard deviation [SD]) or median (interquartile range [IQR]). The t test of the Mann–Whitney U test was used for continuous variables, and the chi-square test was used for categorical variables. Univariate Cox proportional hazards analysis was conducted to compare survival between the conventional and BIA-guided volume control groups at 28 and 90 days after randomization, as well as the aforementioned secondary endpoints. In the secondary analysis, we compared the risk of patient survival according to the achieved volume accumulation rate over 3 days. The volume accumulation rate was calculated using the following equation:
$$Achieved,volume, accumulation, rate = frac{Cumulative,fluid ,balance ,for ,3 ,days ,after, randomization}{{Fluid ,overload ,measured ,by, BIA ,at ,enrollment}} times 100$$
The achieved volume accumulation rate was analyzed as follows: (1) a categorical variable in which the achieved volume accumulation rate was stratified into 4 groups (≤ − 50%, − 50–0%, 1–50%, and > 50%) and (2) a continuous variable in 10-% increments.
The Cox proportional hazards model was used for the analysis. We made incremental adjustments with the following variables: Model 1 is an unadjusted model. Model 2 was adjusted for age, sex, body mass index, type of infection, and intervention arm. We added the VDI, APACHE II score, fluid overload, and serum lactate level to Model 3. In Model 4, the CRRT ultrafiltration rate and CRRT dose were added. The results of Cox proportional hazards regression are presented as hazard ratios (HRs) and 95% confidence intervals (CIs).
Data analyses were performed by an independent statistician blinded to the treatment assignment. P < 0.05 was considered statistically significant.
Ethics approval
This study was approved by the institutional review board of Severance Hospital with registration number 4-2014-0791, and was registered at http://www.clinicaltrials.gov with the trial registration number NCT02384525.
Informed consent
Written informed consent was obtained from all participants and/or substitute decision makers.
- The Renal Warrior Project. Join Now
- Source: https://www.nature.com/articles/s41598-024-64224-z