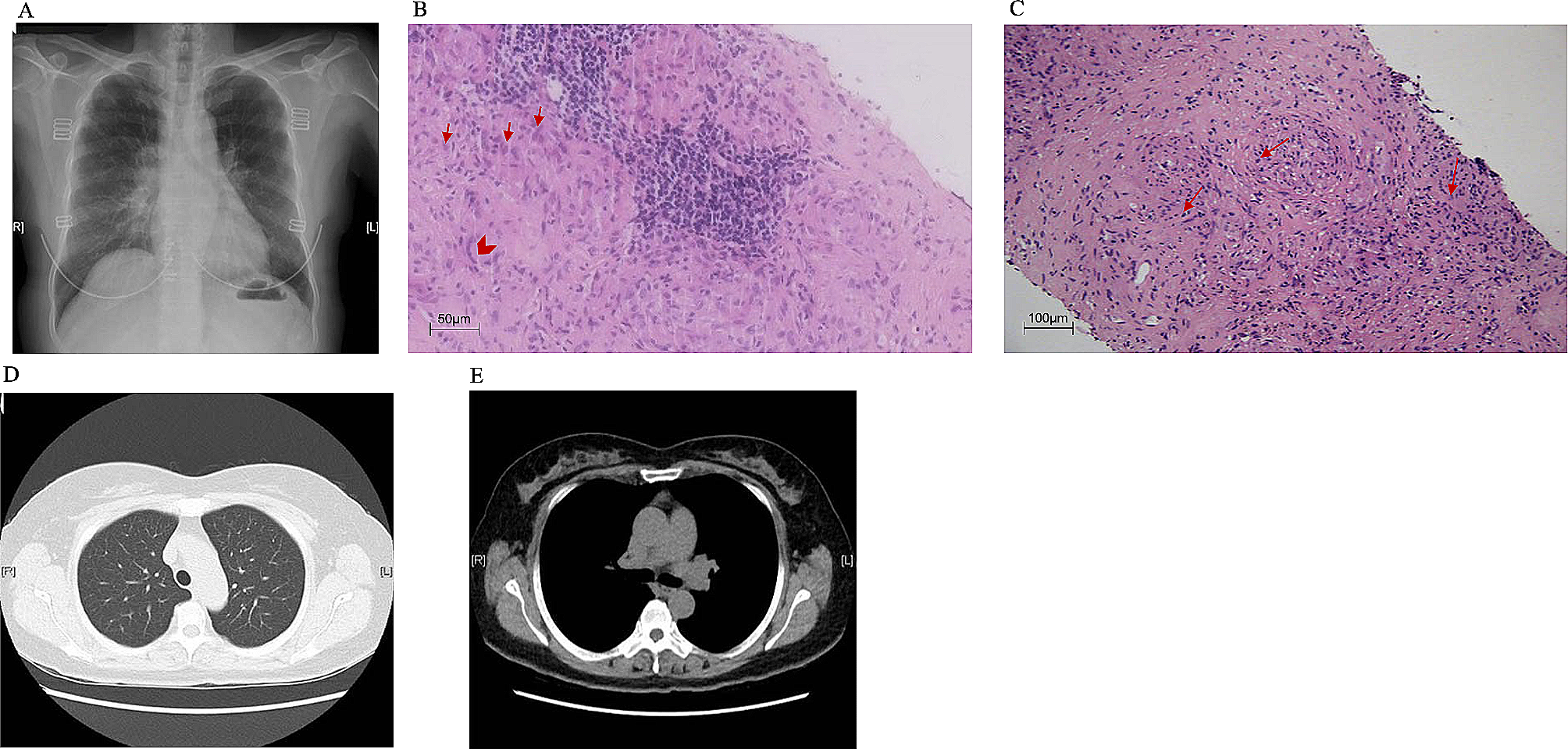
MN is the major cause of nephrotic syndrome in adults. The etiology of approximately 80% of MN cases is idiopathic. Secondary causes include infection, malignancy, autoimmune disease, and drugs/toxins [6]. Anti-PLA2R Ab is the first diagnostic biomarker found in idiopathic MN (iMN), which could be detected in 70% of patients with iMN [7, 13]. Our patient was anti-PLA2R Ab positive, accompanied by renal pathology typical of MN, which strongly supports the diagnosis of PLA2R-associated MN. Nevertheless, secondary causes of MN should also be carefully considered, one of which is sarcoidosis [10].
Sarcoidosis preferentially involves the lung and lymph nodes but also affects other organs [1]. Renal involvement occurs in 10–20% of sarcoidosis cases and most often consists of disorders in calcium metabolism with or without nephrocalcinosis and nephrolithiasis, as well as granuloma formation within the renal interstitium [4]. Although rare, different glomerular diseases have been reported in patients with sarcoidosis, and the most frequent one was MN [4, 5].
The coincidence or causal relationship between MN and sarcoidosis remained unclear. By searching PubMed, 8 articles with full text regarding sarcoidosis and MN were noticed [5, 10, 14,15,16,17,18,19,20], which were summarized in Table 2. Among them, two articles mentioned the relationship between PLA2R and sarcoidosis. Knehtl et al. described for the first time PLA2R-associated MN in the context of sarcoidosis, suggesting a possible relationship between these two diseases [20]. Then, Stehlé T et al. retrospectively reviewed 9 patients with MN without evidence of secondary cause except for sarcoidosis. They found a high prevalence (5/9, 55%) of PLA2R antigens on renal biopsy. Interestingly, all five patients who were positive for PLA2R antigen had active sarcoidosis, and in the available follow-up sera of two patients, anti-PLA2R antibody followed sarcoidosis activity [10]. The above studies revealed that anti-PLA2R-associated MN might be secondary to sarcoidosis, but it is important to note that a causal relationship between the two disorders was found only in patients with active sarcoidosis at the onset of or during the course of MN. In our patient, sarcoidosis is the most suspicious secondary etiology. However, sarcoidosis-associated MN was not plausible in this patient as her sarcoidosis remained resolved at the onset and during the course of MN. Therefore, MN in our patient was inferred to be idiopathic rather than secondary to sarcoidosis.
Even though MN in our patient might not be caused by sarcoidosis, these two rare autoimmune diseases that occurred in the same patient could not be that “coincident”. The underlying molecular link between sarcoidosis and MN remains unclear, as there was no specific antibody or target antigen has yet been identified [21]. The high prevalence of anti-PLA2R antibodies in MN associated with active sarcoidosis indicated that there might be some common predisposing factor in the pathogenesis process in these two diseases.
Interestingly, by searching the genetic background, genetic variations that could increase the susceptibility to both MN and sarcoidosis were found in our patient [1, 3, 6, 11]. HLA-DRB1*0301 and HLA-DRB1*1501 were both risk alleles identified for iMN in the Chinese Han population [11]. HLA-DRB1*0301 was found to be independently correlated with higher anti-PLA2R Ab levels, which plays a major role in MN disease occurrence and antibody production [22]. Besides, a study conducted by Wennerstrom A et al. observed that HLA-DRB1*1501 was a susceptible allele for sarcoidosis and HLA-DRB1*0301 was associated with resolving disease when compared with the persistent group [12]. The above indicated that MN and Sarcoidosis shared common susceptible alleles. In addition, a recent study showed that target antigens detected in sarcoidosis-associated MN reflect the overall incidence of target antigens in MN, in contrast to target antigens in other diseases associated with MN in which a distinctive target antigen has been identified [21], which indicated there exists differences in the relationship between sarcoidosis and MN from other secondary MN. The possible relationship could be a heightened immune response state induced by sarcoidosis in a background of genetic susceptibility which triggered the onset of MN [21]. Moreover, the response to treatment could also be explained by the alleles since HLA allele DRB1*0301, results in high levels of anti-PLA2R Ab and refractory MN [6] but is a favorable allele for good clinical response in sarcoidosis [12].
HLA risk alleles induced a disease-prone context in the patient, but the onset of the disease in a genetically susceptible person is usually triggered by additional insults. Previous studies demonstrated that air pollution and environmental exposures were positively associated with the prevalence of MN and sarcoidosis [23, 24]. The PLA2R mRNA was expressed in the kidney, lung, placenta, liver, and skeletal muscle [25]. It is speculated that environmental factors could damage the lung, and further trigger the production of anti-PLA2R Ab by upregulation of PLA2R expression in macrophage cells in pulmonary alveoli, and eventually develop autoantibody against PLA2R. After a thorough review of the previous history of our patient, a history of long-time metal dust exposure was noted which might contribute to the onset of her sarcoidosis and iMN.
Ultimately, our patients ended up with ESRD, the reason of which was multifactorial. First, this patient’s MN was at a high risk of progressive loss of kidney function due to her massive proteinuria and high level of anti-PLA2R Ab according to the 2021 KDIGO guideline [26]. Second, the patient carries the HLA allele which was independently correlated with higher anti-PLA2R Ab levels, contributing to the refractory to her MN treatment [22]. Third, the patient had been taking traditional Chinese herbs for more than one year, so drug-related kidney injury could not be excluded. Last but not least, the patient had poor adherence, was not followed regularly, and discontinued medication on her own, which participated greatly in her progression to ESKD. Renal ultrasound data at baseline and outcome showed the progression of the disease, with enhanced renal parenchymal echogenicity, which was presented in Supplementary Fig. 1.
In summary, we reported a patient who had both sarcoidosis and anti-PLA2R-associated MN and carried HLA-DRB1*1501 and HLA-DRB1*0301, the risk alleles for both diseases, which provides one more possible explanation for the association between these two diseases.
- The Renal Warrior Project. Join Now
- Source: https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-024-03649-0
