Recently, the number of MN patients in China has significantly increased, with a younger onset age and some patients gradually progressing to end-stage kidney disease after only several years2. Exploring the risk factors of MN onset and timely intervention is crucial; however, there are limited studies on MN with GIL. Most research on ischemic renal diseases focuses on ischemic nephropathy caused by renal artery stenosis. The pathogenesis of ischemic renal diseases may be related to the decrease in renal blood perfusion exceeding the threshold of self-compensation, causing corresponding renal tubular damage, shrinkage of glomerular capillary loops, glomerulosclerosis, and gradual renal atrophy8. Our study found that the prevalence of GIL in MN was not low (37.8%), and there is increasing evidence that glomerular ischemic lesions play an important role in the progression of kidney disease9,10,11.
Hyperuricemia is a part of metabolic syndrome, and the prevalence shows an increasing trend. The prevalence of hyperuricemia in this study was 27.4%, slightly lower than that observed in another single-center study in the same province of China12. This difference might be related to recruiting PMN patients with normal renal function and excluding patients with secondary hyperuricemia. Epidemiology shows that not only is hyperuricemia an independent risk factor for cardiovascular and cerebrovascular events, but it is also a high-risk factor for the progression of chronic kidney disease (CKD)13,14. Previous studies have demonstrated that serum uric acid serves as an independent risk factor for the progression of IgAN, with a more pronounced impact observed among females compared to males15,16. Higher uric acid levels may also contribute to developing global and new renal damage in patients with lupus independently of other known risk factors17,18. Additionally, the latest research shows that hyperuricemia contributes to nephrosclerosis with ischemia and hyperfiltration19. Our study found that the prevalence of hypertension and LDL in the hyperuricemia group was significantly higher than those in the control group (P < 0.05), which was consistent with previous studies20,21. Additionally, we noted that the serum albumin in the hyperuricemia group was higher than that of the control group, which might be related to the richer diet of hyperuricemia patients on the premise that there was no significant difference between the two groups in urine protein quantitation. Hyperuricemia can lead to vasoconstriction and endothelial dysfunction; however, it remains unknown whether hyperuricemia causes GIL. In this study, the prevalence of GIL in the hyperuricemia group (69.1%) was significantly higher than in the control group (26.0%), suggesting that hyperuricemia might increase the risk of GIL. The prevalence of arteriosclerosis in the GIL was found to be significantly higher than that in the non-GIL according to our study findings. However, subsequent binary logistic regression analysis revealed that the arteriosclerosis prevalence did not emerge as an independent risk factor for glomerular ischemia. Although we rigorously selected PMN patients with normal renal function, the estimated glomerular filtration rate (eGFR) of the GIL group, as determined by univariate analysis, was significantly lower compared to that in the non-GIL group. However, subsequent multivariate logistic regression analysis revealed that the association between eGFR and GIL in this study was not statistically significant, suggesting that the decline in eGFR did not substantially impact the statistical outcomes of this investigation. The aforementioned findings may be attributed to the inclusion of patients with normal renal function (eGFR > 90ml/(min.1.73m2), thereby minimizing the impact of eGFR in this study.
The accumulation of uric acid in the kidney stimulates the degranulation of mast cells and the release of renin to promote the production of Ang II, leading to renal oxidative stress, mitochondrial structural damage, and microvascular system damage22. The presence of hyperuricemia is associated with the activation of both plasma renin and intra-kidney angiotensin activities, leading to renal vasoconstriction and potentially inducing ischemia in the kidney23. Furthermore, hyperuricemia causes oxidative stress and inflammation, which leads to the impairment of nitric oxide (NO) synthase (NOS) and consequent local vasoconstriction, ultimately leading to glomerular ischemia24. As mechanisms of CKD progression due to hyperuricemia, endothelial dysfunction induced by uric acid and activation of the Nod-like receptor protein 3 (NLRP3) inflammasome induced by urate crystals have been a focus of research25,26. Recently, ROS/NLRP3 inflammasome-mediated endothelial cell pyroptosis has been reported as the critical mechanism underlying the relationship between hyperuricemia and atherosclerosis27. Previous studies have shown that uric acid can induce the loss of glycocalyx in endothelial cells, which may be one of the mechanisms of endothelial dysfunction and kidney diseases28. The absence of the glycocalyx is the earliest sign of endothelial damage, leading to increased vascular permeability and a higher risk of intravascular thrombosis, resulting in microcirculation dysfunction, organ ischemia, and organ injury29. Recent studies have demonstrated a consistent association between hyperuricemia and renal damage, which is frequently attributed to the presence of insulin resistance (IR). In the context of hyperuricemia, elevated levels of uric acid significantly disrupt insulin signaling pathways, thereby diminishing insulin-induced eNOS activation and expression as well as NO synthesis in endothelial cells. Consequently, this cascade culminates in the development of endothelial cell IR, ultimately leading to impaired NO-dependent vasodilation and endothelial dysfunction that exacerbate renal ischemia30.
In the current study, we found that TA/IF was closely related to GIL. A conventional perspective on kidney diseases emphasizes the glomerular origin of disease progression, which initiates as glomerulosclerosis and subsequently induces hypoxia in the tubular segments. This leads to further tubular atrophy and interstitial fibrosis31. The latest evidence suggests that tubular interstitial damage may also contribute to an increased extent of glomerular damage32. Tubular interstitial fibrosis can induce tubule-to-glomerulus injury through the mechanism of tubuloglomerular crosstalk, thereby accelerating the progression of renal disease33. In human posttransplant kidneys, a follow-up of the development of glomerulosclerosis identified both interstitial fibrosis and tubular atrophy as independent predictors of subsequent glomerulosclerosis34. We conducted additional statistical analysis and categorized the study participants into two groups, namely the IF group and non-IF group, based on the results of kidney biopsy. Binary logistic regression analysis was performed on five variables that showed significant associations in univariate analysis: age, serum uric acid level, eGFR, serum albumin, and presence of arteriolosclerosis. The findings demonstrated that serum uric acid level remained an independent risk factor for GIL in the TA/IF group, while both serum uric acid level and eGFR were identified as independent risk factors for GIL in the non-TA/IF group. These results provide additional evidence supporting the role of uric acid in glomerular ischemia (refer to supplementary materials Tables S1–S2).
This study had several limitations. First, serum uric acid level was only measured once, without long-term follow-up data, resulting in some selective biases. Second, unintentional biases may have been introduced due to the cross-sectional retrospective design of this study in combination with a relatively small sample size. Multi-center, large sample, and RCT studies are required for further validation.
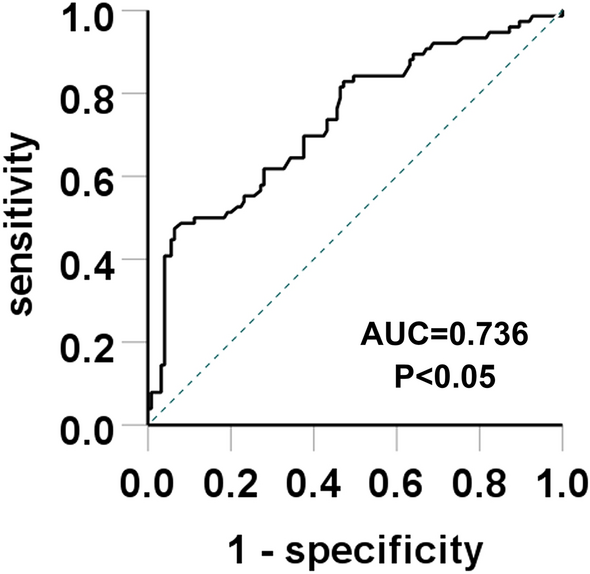
In summary, this study included 201 cases of PMN. The prevalence of GIL in the hyperuricemia group was higher than in the control group. Multivariate logistic regression analysis indicated that serum uric acid level and TA/IF off patients were independent risk factors for GIL. According to the ROC curves, the increase of uric acid showed predictive values in predicting GIL. When the serum uric acid level was greater than 426.5 μmol/L, the prevalence of GIL also increased. Therefore, clinicians and other medical professionals should focus on controlling serum uric acid levels in PMN patients. However, selecting the correct treatment to reduce GIL requires long-term follow-up and further research.
- The Renal Warrior Project. Join Now
- Source: https://www.nature.com/articles/s41598-024-57813-5
