Ethical consideration and informed consent
This study adhered to the principles of the Helsinki Declaration and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement. Ethical principles of autonomy, confidentiality, and anonymity were considered. The ethical review board at the Institute for Endocrine Science, Shahid Beheshti University of Medical Science approved the study with the ethical number of IR.SBMU.ENDOCRINE.REC.1401.069. Medical staff from the research team thoroughly explained the study protocol to each participant individually. Written informed consent was obtained from all participants, and in cases of refusal, the participant was excluded from the study. The informed consent included permission for data collection, performing the required laboratory tests, and publication while maintaining confidentiality principles.
Tehran lipid and glucose study
This prospective cohort study is based on the Tehran Lipid and Glucose (TLGS) cohort, which is a longitudinal population-based cohort aimed at investigating non-communicable diseases in Tehran, Iran. TLGS study included fifteen thousand adult residents in the eastern area of Tehran, Iran using a multistage cluster random sampling method. Participants were followed every three years, and data was collected from 1999 to 2021. The full details of TLGS were previously described19,20.
All participants invited to the TLGS unit are referred to experienced physicians after providing their informed written consent. These physicians conduct interviews to gather participants’ past medical history and complete a comprehensive 110-item questionnaire. This questionnaire covers a range of topics, including family history of NCD, smoking habits, reproductive history, and physical activity assessment. A brief physical examination, including anthropometric measurements, is also performed. Dietary data for one-tenth of the participating families is collected by trained dietitians.
Education levels are categorized into three groups: primary (up to 6 years), secondary (6–12 years), and tertiary education (over 12 years). Physical activity levels are quantified using METS derived from activity questionnaires, with less than 600 min/week indicating low activity. Physicians trained in obtaining anthropometric measurements record WC, weight, and height following standard protocols, and BMI is calculated accordingly. Participants are asked to remain seated for 15 min, after which a qualified physician measures blood pressure twice using a standard mercury sphygmomanometer calibrated by the Iranian Institute of Standards and Industrial Researches.
Biochemical analysis
Upon admission, personal characteristics are documented, and a unique computer code is assigned. A 10-mL sample of venous blood is collected from all study participants between 7:00 and 9:00 a.m. after a 12–14 h overnight fast. The blood samples are taken in a sitting position following a standard protocol and are kept for one and a half hours under standard lab conditions.
All laboratory kits are provided by Pars Azmon Inc., Iran. Serum total cholesterol and triglycerides (TG) are measured using enzymatic calorimetric tests with cholesterol esterase and cholesterol oxidase, and glycerol phosphate oxidase, respectively. HDL is measured after the precipitation of apolipoprotein B containing lipoproteins with phosphotungistic acid. Assay performance is monitored every 20 tests using the lipid control serum, Precinorm [normal range] and Precipath [pathologic range] (Boehringer Mannheim, Germany; cat. no. 1446070 for Precinorm and 171778 for Precipath).
Serum glucose concentration is assayed using an enzymatic colorimetric method with a glucose oxidase technique to assess fasting blood glucose (FBS). Assay performance is monitored every 20 tests using the glucose control serum, Precinorm [normal range] and Precipath [pathologic range] (Boehringer Mannheim, Germany; cat. no. 1446070 for Precinorm and 171778 for Precipath). A glucose standard (C.f.a.s, Roche, Germany; cat. no. 759350) is used to calibrate the Selectra 2 auto-analyzer on all days of laboratory analyses. All samples are analyzed when internal quality control meets the acceptable criteria. Inter- and intra-assay coefficients of variations are both 2.2% for serum glucose and 0.6% for TGs19. Serum creatinine (Cr) is measured according to the standard colorimetric Jaffe-Kinetic reaction method (Pars Azmon Inc, Tehran, Iran).
Study design and exclusion criteria
In this study, we collected available data for demographic data (age, sex), laboratory exams (FBS, HDL, TG, Cr), smoking status, education, physical activity, and hemodynamic indicators (systolic and diastolic blood pressure) from the accessible TLGS database. Participants completed the sixth round of follow-up every three years, resulting in a minimum of 18 years of prospective observation.
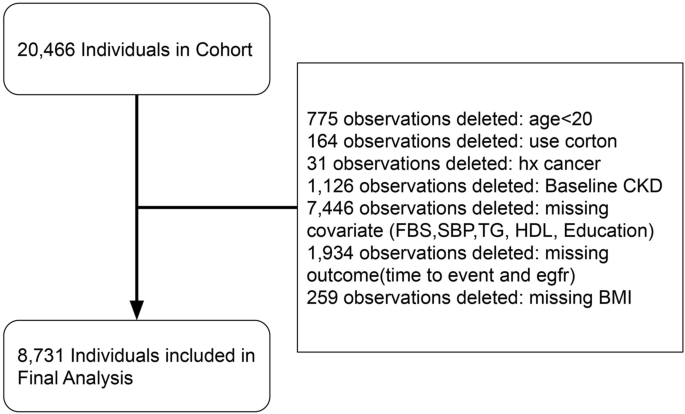
We selected participants for our study from the pool of participants in the TLGS cohort based on the following criteria: availability of valid data in phases of the TLGS cohort, and exclusion of participants who had missing values in covariates or target variable of glomerular filtration rate (GFR), were under 20 years old, diagnosed with cancer during the study period, or received corticosteroid treatments (Fig. 1). From this enrolled cohort, we excluded participants who met any of the following criteria: (1) eGFR < 60 mL/min at baseline; (2) follow-up duration of less than one year; (3) lack of conclusive data on metabolic and renal function.
Aim and outcome
This research aimed to answer the following questions:
-
Primary Objective 1: Evaluate the risk of CKD incidence among four obesity-metabolic phenotypes.
-
Primary Objective 2: Determine the impact of participants’ state transitions on CKD risk.
-
Extended Investigation 1: Assess the adjusted effect of each component of metabolic health on CKD risk.
-
Extended Investigation 2: In the context of defining obesity-metabolic health states, what is the impact of the number of metabolic health components, with or without obesity, on CKD risk?
-
Extended Investigation 3: In terms of defining obesity-metabolic health states, what is the concordance between using WC and BMI for defining obesity?
The main outcome of study was the occurrence of CKD, defined as having two GFR < 60 mL/min/1.73m2 in two phases of TLGS cohort which have three years interval. The CKD-EPI equation was used for GFR calculation, which is as follows21:
$$GFR=141 times {{text{min}}(frac{Scr}{k}, 1)}^{alpha } times {{text{max}}(frac{Scr}{k}, 1)}^{-1.209}times {0.993}^{Age}times 1.018[if ;female]$$
where Scr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is -0.329 for females and -0.411 for males, min indicates the minimum, and max indicates maximum.
Definition of metabolically health, obesity, and four phenotypes of their interplay
The definition of metabolic health varies across studies and is subject to debate15. In this study, we utilized our previously validated definition of metabolic health, which is based on an evidence-based approach combined with expert opinions using the Delphi method15. The consensus among experts to exclude Waist Circumference (WC) from the metabolic health definition aligns with the National Cholesterol Education Program Adult Treatment Panel III22. Consequently, the metabolically unhealthy condition in this study was defined as the presence of three or four of the following criteria:
-
(1)
Decreased HDL (< 40 mg/dL in men or < 50 mg/dL in women).
-
(2)
High TG (≥ 150 mg/dL).
-
(3)
High FBS (≥ 100 mg/dL) or use of anti-glycemic oral medication for glycemic control.
-
(4)
Systolic blood pressure equal to or above 130 mmHg OR diastolic blood pressure equal to or above 85 mmHg or antihypertensive medication.
Obesity was defined as BMI greater than 30 kg/m2. For the purpose of investigating the concordance of obesity-metabolic health and WC-metabolic health, we defined abnormal WC as ≥ 95 cm, according to the cutoff points for Iranian adults of both genders23. Based on the state of obesity and the presence of metabolic syndrome components, participants were categorized into four major groups:
-
MH-NO Phenotype: This refers to individuals who exhibit a metabolically healthy profile and do not have obesity (Metabolically Healthy, No Obesity: MH-NO).
-
MU-NO Phenotype: This category includes individuals who have a metabolically unhealthy profile but do not have obesity (Metabolically Unhealthy, No Obesity: MU-NO).
-
MH-O Phenotype: This group consists of individuals who have a metabolically healthy profile but have obesity (Metabolically Healthy, Obesity: MH-O).
-
MU-O Phenotype: This phenotype pertains to individuals who have a metabolically unhealthy profile and also have obesity (Metabolically Unhealthy, Obesity: MU-O).
Statistical analysis
The statistical analysis was performed using Stata (StataCorp. 2015. Stata statistical software: Release 14, College Station, TX: StataCorp LP.). A p-value of less than or equal to 0.05 was considered statistically significant. The baseline characteristics of participants were expressed as mean, standard deviation, median, and interquartile range (IQR) for continuous variables and frequency (%) for ordered variables. The baseline characteristics of participants between four groups were compared by the Student’s t-test and Chi-square test for continuous and ordered variables, respectively. We used the Cox proportional hazard test to analyse the association between obesity phenotype and metabolic unhealthy profile with CKD. We defined survival time as the interval between study inclusion and CKD advent or censoring. Also, the event time was defined as half-time survival between the first diagnosis of CKD and the last normal laboratory result. The univariate Cox regression analysis was performed for all confounding factors such as age, sex, BMI, and smoking.
Additional variables with a p-value of less than 0.2 in the univariate study were considered for multivariate regression model analysis. The first statistical model (unadjusted) showed crude rates. The second model (age-sex adjusted) was adjusted for age and sex. The third model (fully adjusted) was adjusted for age and sex (female, male), and the third analysis was adjusted for age, sex, smoking (smoker, non-smoker), education (primary education, college undergrad, postgrad), and physical activity (Metabolic Equivalent of Task < 600, > 600). The Schoenfeld residual test was used to examine the proportional hazard assumption in the Cox model. The cumulative incidence rate of CKD was calculated as new cases divided by the at-risk individual-time during the follow-up period. Additionally, the effect of each component on the total cohort was evaluated after adjusting for covariates and other components.
Finally, three hazard ratios and their corresponding 95% confidence interval (presented as HR [lower confidence interval, upper confidence interval] of CKD incident were calculated using three Cox tests: (1) Unadjusted, (2) Age and sex adjusted, (3) Fully adjusted (called as adj-HR) (age, sex, smoking, education, physical activity). For reports of HRs stratified by gender, the stratified variable was excluded from the adjusted models.
- The Renal Warrior Project. Join Now
- Source: https://www.nature.com/articles/s41598-024-56061-x