WHAT IS ALREADY KNOWN ON THIS TOPIC
-
Previous randomised controlled trials have shown the efficacy and safety of fixed-dose combination therapy containing statins, antihypertensive agents, with or without aspirin for primary and secondary prevention of cardiovascular diseases (CVDs). The wider application of such interventions to middle-aged and older patient groups in typical rural settings is uncertain.
WHAT THIS STUDY ADDS
-
This large-scale pragmatic cluster-randomised controlled study provided evidence for the effectiveness and safety of fixed-dose combination therapy for primary and secondary prevention of CVD in a rural population from south Iran. No safety concerns emerged despite low achieved blood pressure levels.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
-
An inexpensive and affordable polypill can be used for both primary and secondary prevention at the community level, especially in low-resource settings. This calls for wider adoption of this intervention.
Introduction
Cardiovascular diseases (CVDs) are major causes of mortality and morbidity worldwide, with an estimated 19 million deaths in 2019, a 58% increase in number of deaths since 1990.1–3 Despite the availability of non-pharmacological and pharmacological interventions, health systems around the world have not been successful in preventing CVDs. There is accumulating evidence on effectiveness and safety of fixed-dose combination therapies (or polypills) in CVD prevention in selected low-income and middle-income countries.4–6 However, the majority of the existing evidence is focused on secondary prevention of CVDs.
Recently three large-scale randomised controlled trials were conducted on the effectiveness and safety of polypills. The PolyIran Study established in northeastern Iran demonstrated the effectiveness and safety of a polypill in both primary and secondary prevention.7 The HOPE-3 and TIPS-3 clinical trials mainly aimed to investigate the effectiveness of polypill strategy on primary prevention of CVDs in a number of mainly low and middle-income countries.8–10 All of these three trials showed a higher adherence to polypill and a substantial reduction in CVD events. An individual participant data meta-analysis on the three trials confirmed the results of individual trials, showing higher effectiveness of strategies including aspirin compared with strategies without aspirin.11
Despite the burgeoning body of randomised evidence, questions persist regarding the potential variability of polypill effectiveness across communities with differing ethnicities, cultures, socioeconomic statuses and lifestyles, and in the absence of intensive medical care for monitoring outcomes. The present trial, PolyPars, was undertaken to explore the efficacy and safety of the polypill for CVD prevention in a rural population in the southern regions of Iran, aiming to provide insights into its applicability across diverse demographic settings.
Methods
Study design, setting, inclusion and exclusion criteria
The PolyPars Study was a two-arm pragmatic cluster-randomised controlled trial nested within the Pars Cohort Study (PCS) in south of Iran, in which 9264 participants were enrolled. The methods and design of the PCS and PolyPars Studies have been described previously.12 13 All residents in the entire district of Valashahr aged 40–75 years were recruited in PCS from 2012 to 2014. All participants in the PCS older than 50 years in 2015 were invited to take part in PolyPars. Two arms were defined in PolyPars. The control arm was designed to receive lifestyle modification advice, and the intervention arm was designed to receive the polypill in addition to the advice. Participants who accepted the invitation and referred to the PCS centre were informed about the trial, and upon agreeing to participate, the PolyPars team obtained written informed consent. After the initial enrolment, the eligibility of the participants was assessed based on predefined criteria and all eligible participants received the lifestyle advice at this stage. Exclusion criteria included diseases preventing compliance, contraindications to polypill components or unwillingness to participate, leading to 589 participants being excluded as detailed in online supplemental table 3.
Supplemental material
Following the enrolment of eligible participants, villages, which served as the unit of study, were randomised into intervention (46 clusters) and control arms (45 clusters) to prevent medication sharing within villages, thereby ensuring that interventions were applied at the cluster level. We divided villages into three groups based on their population size. Block randomisation was used for each group of villages and a computer-generated list of numbers was obtained. An independent statistician in the National Cancer Institute, who was blinded to recruitment of participants, produced the random allocation sequence. While blinding the PolyPars team and the participants was not possible, the outcome assessors and the analytical team remained unaware of the group allocations. Participants in the intervention arm were re-invited to receive the medication along with instructions for its use. Following explanation of the study procedures, the components of the polypill, its potential benefits and possible adverse events, the PolyPars team obtained additional written informed consent from all participants. The trial was registered with ClinicalTrials.gov (NCT03459560).
Data collection
The PolyPars team at the PCS centre documented demographic characteristics, participants’ medical and medication histories, and measured anthropometric indices. At baseline, blood pressure was measured once in a standing position and twice in a sitting position, with the mean of the two sitting measurements being used for analyses. Biannual measurements were conducted during the follow-up, with participants diagnosed with hypertension referred to primary care practice. A blood sample was collected after ensuring that the participant had fasted for at least 8 hours. Data for this study were anonymised by removing personal identifiers and assigning unique IDs, with all related documents securely stored in a safe place at the PCS centre.
Interventions
Participants in both study arms were provided with a non-pharmacological intervention package, encompassing educational training on a healthy lifestyle, covering aspects like diet, physical activity, weight management, and cessation of smoking and opium use, delivered through illustrated pamphlets and detailed verbal consultations. Control group participants meeting the national criteria for hypertension were referred to the local primary healthcare practice for blood pressure management, a service nationally available to the entire community free of charge.14 In the polypill arm, two formulations were prescribed. Participants were at first prescribed polypill one, which included hydrochlorothiazide 12.5 mg, aspirin 81 mg, atorvastatin 20 mg and enalapril 5 mg. Participants who reported cough during the follow-up were switched to polypill two, which included valsartan 40 mg instead of enalapril. For patients already on any polypill components or other lipid-lowering, blood pressure-lowering or antiplatelet medications, their doses were adjusted in addition to the polypill by the trial’s physician. Since the polypill’s component dosages are typically lower than what patients require, any additional necessary dosage not provided by the polypill is prescribed alongside it.
Follow-up
Participants were followed up by two independent groups: the field follow-up team (responsible for follow-up visits) and the central follow-up team (responsible for outcome ascertainment). At each follow-up visit, participants in the polypill group were asked to bring their empty blisters to the next visit. The field follow-up team interviewed participants to maintain study participation and document the presence of any symptoms indicating adverse events. Follow-up visits for the control group were scheduled at 3 and 6 months, while the polypill group had visits at 1, 2, 3 and 6 months, with both groups subsequently followed every 6 months after the initial 6-month period.
The central follow-up team was responsible for ascertainment of outcomes. This team was independent of the field follow-up team and was blind to participant allocation and adherence. The study stopped 60 months after the recruitment of the last participant.
Outcomes
The primary outcome of interest was the composite of the first occurrence of major cardiovascular events, which included hospitalisation for acute coronary syndrome (non-fatal myocardial infarction and unstable angina), fatal myocardial infarction, non-fatal and fatal stroke, sudden death and heart failure. If more than one major cardiovascular event was reported, the first event was considered for the analysis. Secondary outcomes were the individual component of primary outcome, all cardiovascular deaths, deaths from non-cardiovascular causes, overall mortality and change in mean of blood pressure. Upon identifying any cardiovascular event, a member of the central follow-up team gathered all pertinent medical documents from hospitals or the patients’ homes. Two independent and blind internists reviewed all documents and ascertained the outcome. In cases of discordance, the outcome was determined by a third senior internist.
Study sample size
Considering the major cardiovascular event rate of 0.0182 per annum in PCS, we approximated the 5-year risk to be about 0.088. With 91 clusters, an intracluster correlation of 0.005 derived from the prior trial in northeast part of Iran and a coefficient of variation of cluster sizes of 0.9, if 1620 participants were enrolled in each arm, the study would possess an estimated 80% power (at a 5% significance level) to detect a relative risk of 0.65. Considering the 20% dropout rate, the total required sample size would be 2025 participants in each arm.
Statistical analyses
We performed intention-to-treat analyses using Cox regression models, with shared frailty to account for cluster randomisation. We estimated both adjusted and unadjusted HRs and 95% CIs. In the adjusted model, the following variables were included: age, sex, diabetes mellitus, hypertension and history of major cardiovascular events. The number needed to treat (NNT) was estimated using the inverse of absolute risk reduction, considering the minimal intracluster correlation observed in the study.
Follow-up time was estimated since the eligibility visit. Follow-up was censored at the time of loss to follow-up, occurrence of the primary outcome, death from non-major cardiovascular causes or the end of the study (60 months), whichever came first. We performed several prespecified subgroup analyses by sex, age group, pre-existing hypertension, pre-existing diabetes mellitus, history of smoking, baseline cholesterol and pre-existing CVDs. For the estimation of effect size for each subgroup, interaction terms were incorporated into the models.
An interim analysis was performed 2 years after enrolment of the last participant and the blinded results were given to the data monitoring committee (DMC). DMC members met online at 6-month intervals and two members in Iran travelled to the study field every 6 months and continuously monitored the trial in terms of any protocol deviation or any harm. The DMC members reached an agreement on continuation of the study to complete the 5-year follow-up. Statistical analyses were done using Stata V.11 software.
Results
Out of the 9264 participants from the original cohort, all 5430 individuals aged over 50 years were invited. A total of 5004 participants responded, while 426 invitees did not attend the centre. There was no difference in the demographic characteristics between the referring and non-referring invitees. Out of the entire referring invitees, 589 participants were ineligible for participation and their characteristics are shown in online supplemental tables 1–3. The flow chart of participant enrolment is demonstrated in figure 1. The average number of participants per cluster was 48.5. In 751 participants, the type of the pill was changed to type two due to cough.
” data-icon-position data-hide-link-title=”0″>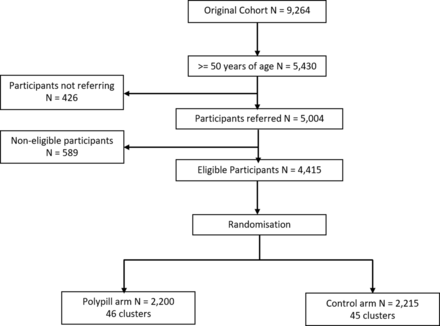

Flow chart of participant recruitment in PolyPars.
From December 2015 to December 2016, a total of 4415 participants were recruited, 2200 participants in the polypill arm and 2215 participants in the control arm. The baseline characteristics are provided in table 1. The mean age of participants was 59.9 years (SD 6.7). About 55.0% of the participants were female. The two study groups were similar with respect to potential confounders (online supplemental table 4). A total of 713 (16.1%) of 4415 participants had a history of CVD based on self-report and 552 (77.4%) of these were using non-trial antihypertensive medications at baseline, 352 (49.4%) used lipid-lowering medications and 544 (76.3%) used non-trial aspirin. The use of non-trial CVD medicines was similar between the study arms, with or without pre-existing CVD (table 1). The median of follow-up duration was 4.6 years (IQR 4.4–4.9).
Baseline characteristics of participants in polypill and control arms
During follow-up, 176 (8.0%) of 2215 participants in the control group developed the primary outcome compared with 88 (4.0%) of 2200 participants in the polypill group (adjusted HR 0.50, 95% CI 0.38 to 0.65; figure 2). The absolute risk reduction was 4.0% (95% CI 2.5% to 5.3%). The 5-year NNT to prevent one major cardiovascular event was 25. In subgroup analysis, we found no difference in the relative effect size (figure 3). Similarly, we observed no heterogeneity of effect between participants who had pre-existing CVD (HR 0.47, 95% CI 0.26 to 0.84) and those who did not have pre-existing CVD (HR 0.50, 95% CI 0.37 to 0.68, p for interaction=0.85) (figures 3 and 4). Adjusting for non-trial cardiovascular drugs taken during the trial did not alter the HR (adjusted HR 0.50, 95% CI 0.37 to 0.68).
” data-icon-position data-hide-link-title=”0″>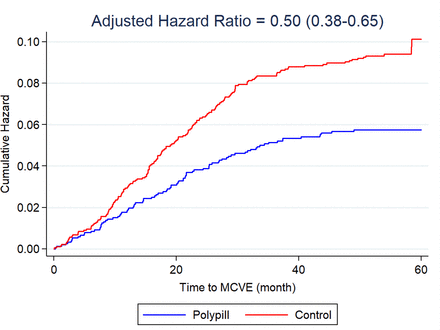

Kaplan-Meier curves for the composite outcome of hospitalisation for acute coronary syndrome (non-fatal myocardial infarction and unstable angina), fatal myocardial infarction, non-fatal and fatal stroke, sudden death and heart failure. Kaplan-Meier curves are presented up to 5 years. MCVE, major cardiovascular event.
” data-icon-position data-hide-link-title=”0″>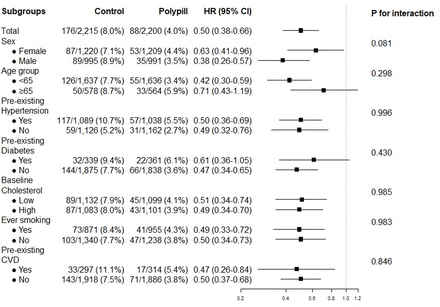

Major cardiovascular events in polypill and control groups. Data are n/N (%) unless otherwise indicated. We obtained HRs and 95% CIs by Cox regression models with shared frailty. CVD, cardiovascular disease.
” data-icon-position data-hide-link-title=”0″>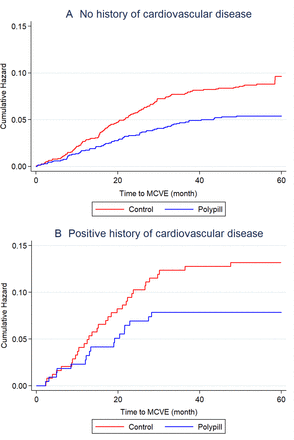

Kaplan-Meier curves for the composite outcome of hospitalisation for acute coronary syndrome (non-fatal myocardial infarction and unstable angina), fatal myocardial infarction, non-fatal and fatal stroke, sudden death and heart failure. Kaplan-Meier curves are presented up to 5 years. (A) Curves in participants with no history of cardiovascular disease. (B) Curves in participants with a positive history of cardiovascular disease. MCVE, major cardiovascular event.
For secondary outcomes, the risk of non-fatal CVD events was significantly lower in the polypill group compared with the control group (adjusted HR 0.47, 95% CI 0.34 to 0.65). We found no differences in the risk of CVD mortality, non-cardiovascular mortality and overall mortality between the study arms (figure 5). The results showed a significant decline in both systolic and diastolic blood pressure in the polypill group at month 24 (mean difference of 5.6 mm Hg (95% CI 3.8 to 7.4) and 3.4 mm Hg (95% CI 2.3 to 4.5), respectively). The decline in systolic and diastolic blood pressure was not significant in the control group at month 24 (mean difference of 0.1 mm Hg (95% CI −1.8 to 2.0) and 0.4 mm Hg (95% CI: −0.7 to 1.5), respectively). However, between month 24 and month 60, the reductions in both systolic and diastolic blood pressures were greater in the control group compared with the polypill group. Therefore, the difference between the two groups was not significant at month 60 (figure 6).
” data-icon-position data-hide-link-title=”0″>

Risk of secondary outcomes in polypill and control groups. Data are n (%) unless otherwise indicated. We obtained HRs and 95% CIs by Cox regression models with shared frailty. CVD, cardiovascular disease; MI, myocardial infarction.
” data-icon-position data-hide-link-title=”0″>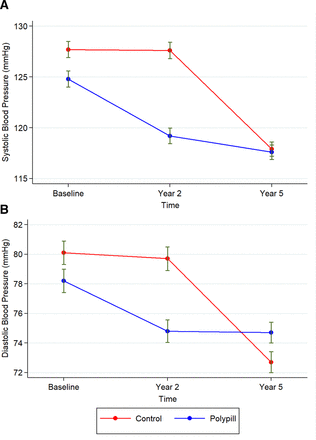

Changes in systolic blood pressure (A) and diastolic blood pressure (B) with a fixed-dose combination treatment strategy compared with control. The figure represents the raw estimation of the mean and 95% CIs of blood pressure at each time point.
Online supplemental table 5 demonstrates the frequency of adverse events in the polypill and control groups. The frequency of muscle pain, dizziness, dyspepsia, regurgitation of food or liquid and heart burn based on self-report was higher in the polypill group. No fatal or non-fatal renal failure was detected throughout the follow-up. Only seven cases of death due to intracranial haemorrhage was reported, four in the polypill group and three in the control group. No gastrointestinal bleeding was reported in this study, which shows the safety of aspirin in polypill.
Discussion
The results of this trial showed that daily administration of polypills over a 5-year period can decrease the risk of major cardiovascular events by approximately 50% in participants aged 50–75 years, with an NNT of 25. No heterogeneity of effect was observed across various subgroups. Despite a higher frequency of non-serious adverse events in the polypill group, serious adverse events were comparable between the two groups, suggesting that polypill administration could safely reduce cardiovascular outcomes.
The current study demonstrated a 33% reduction in CVD mortality. However, despite the previous findings from the northeast part of Iran, this reduction did not reach statistical significance. This controversy may be due to lower mortality rate caused by CVDs in the PARS cohort or better access to healthcare services in this rural community.13 The safety of aspirin for the primary prevention of CVDs is still controversial, specifically due to bleeding side effects.6 Results of PolyIran and the individual participant data meta-analysis showed no significant adverse event in case aspirin was included in polypill.7 11 In line with our findings, the results of the individual participant data meta-analysis further showed that strategies with aspirin are much more effective than strategies excluding aspirin.11
Polypill strategy is affordable and feasible in most parts of the world.15 16 So far, various formulations of polypill have been used worldwide in several studies,17 18 and marketed in different countries, including Iran.19 20 Availability and affordability could be further increased through the inclusion of CVD medications in programmes, such as WHO prequalification programmes. Although polypill can be delivered using separate medications, their combined use in a pill would certainly result in higher adherence.21 A complementary strategy is to improve access to CVD prevention through non-physician healthcare workers and to integrate community-based screening and treatment of CVD risk factors into primary care. The value of such an approach has been shown in the HOPE-4 and PolyIran trials.7 22 This strategy can be effective in different communities to achieve the United Nations Sustainable Development Goals of reducing premature mortality from non-communicable disease by one-third until 2030.23
Many published trials on polypills for atherosclerotic CVD have had short follow-up duration and small to moderate sample sizes. Recent systematic reviews showed the long-term effects of the various formulations of polypill particularly in primary prevention settings.21 24 The large scale, the population-based design and the long follow-up period of the current study are among the advantages that have yielded significant results on effectiveness and safety of polypill in this study. The PolyPars Study has certain limitations as well. First, we used only one type of pill for all participants for both primary and secondary prevention. Flexible doses may improve drug effectiveness. However, fixed-dose combinations are more practical. Second, the PolyPars Study was conducted in a rural community; therefore, generalising the findings to urban populations warrants further investigation.
Finally, in this study, the effect was null for several secondary outcomes. This findings could be attributed to the relatively short follow-up period of 5 years and the low rate of CVD mortality in this region, which may have resulted in our study being underpowered to detect a reduction in mortality. Further studies with longer follow-up are needed to evaluate the effectiveness of polypill for reducing mortality.
In conclusion, the utilisation of a four-component polypill proved effective in substantially reducing the risk of major cardiovascular events in a pragmatic, community-based trial. This trial furnishes essential evidence supporting the integration of a polypill strategy into preventive programmes aimed at mitigating the burden of CVDs, particularly in low-income and middle-income countries.
Data availability statement
Data are available upon reasonable request. The PolyPars Study data including patients’ demographic data, outcome data, covariates data and a data dictionary will be available to other researchers after publication of the main paper. Only de-identified data will be available. The PolyPars data are stored in the internal server of the Digestive Diseases Research Institute at Tehran University of Medical Sciences (Tehran, Iran) and the Shiraz University of Medical Sciences. Data will be provided to researchers according to a research proposal. After approval of the research proposal by the principal investigators of the study, the data will be provided to the researcher after signing of a data access agreement.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Ethics Committees of Shiraz University of Medical Sciences and Tehran University of Medical Sciences (IR.TUMS.DDRI.REC.1395.13). Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We acknowledge the community healthcare staff (Behvarz) for their role in recruitment and follow-up of participants. We also thank the participants for their continued cooperation with this trial.
- The Renal Warrior Project. Join Now
- Source: http://heart.bmj.com/cgi/content/short/110/14/940?rss=1
