Embriology and anatomy
Ebstein’s anomaly (EA) is a congenital valvular and ventricular dysplasia of the right-sided heart, frequently associated with left-sided heart anomalies.1 2 EA results from incomplete delamination of the tricuspid valve (TV) from the right ventricular (RV) endocardium, which occurs between the 7th and 12th week of intrauterine life.3 During this process, the innermost layer of the ventricular wall separates from the underlying tissue, forming TV leaflets and tendinous chordae. Genetics and environmental factors supposed to be involved in EA pathogenesis are provided in online supplemental material (section A, table 1).
Supplemental material
Impaired fibrous transformation from the muscular precursor results in adherence of TV components to the RV wall, thus causing an apical displacement of the septal leaflet (SL) hinge point.1 The same pathological process may involve the posterior leaflet (PL) while less frequently the anterior leaflet (AL), due to a supposed different embryology.1 3 Leaflets may adhere to the endocardium either directly, through linear (figure 1A, C and D; online supplemental video 1) or focal attachments (online supplemental ideo 2), or indirectly, through accessory fibrous chordae or muscular stumps (figure 1C and D; Online supplemental video 1).1
Supplementary video
Supplementary video
” data-icon-position data-hide-link-title=”0″>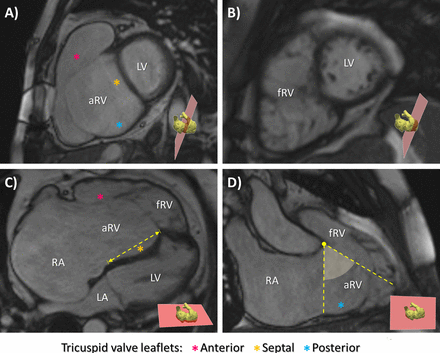

Magnetic resonance cine-SSFP images in a patient with Ebstein’s anomaly in short axis (A, B) and long axis (C, D) views at end-diastole, highlighting the orientation of each cine-SSFP slice. Focusing on the tricuspid valve, apical displacement of the septal leaflet and rotation angle are reported in horizontal long-axis (C) and vertical long-axis views (D), respectively. RV, right ventricle; SSFP, steady-state free precession .
Leaflets adherence to RV wall determines two key EA anatomopathological features: (1) the TV effective orifice separates from the anatomical annulus and rotates towards the right ventricular outflow tract (RVOT) of a degree which depends on the extension of the delamination defect (figure 1C and D)4; (2) the myocardial wall above the effective TV orifice is ‘atrialized’ (figure 1A, C and D), thus rendering EA a ‘RV myopathy’ beyond a valve disease.1 5
The atrialized RV (aRV) lacks of myocardial fiber concentration and presents reduced wall thickness (figure 1A, C and D). The part of RV responsible for stroke volume (SV) generation is the functional RV (fRV), which extends from the effective TV orifice to the pulmonary valve (figure 1B, C and D).1 5 The combination of aRV and fRV identifies the anatomical RV (ARV).
Multimodality imaging
The quantitative criterion for EA diagnosis is an apical displacement of the SL hinge point by at least 8 mm/m2 from the anterior mitral leaflet insertion, assessed in four-chamber view1 6 (figure 1B). An absolute distance in atrioventricular valves offsetting of 15 mm in children and 20 mm in adults is also considered diagnostic.7 Multimodality imaging is pivotal not only for anatomical and functional assessment of TV and right-sided chambers but also for identifying associated lesions (online supplemental material, section B
Transthoracic echocardiography (TTE) is usually the first diagnostic tool. Second-line imaging includes transoesophageal echocardiography (TOE) and cardiovascular magnetic resonance (CMR), the former with particular usefulness in TV assessment, the latter in right-sided chamber volumes calculation and myocardial characterization. CMR is superior to TTE in the detection of PL and extra-cardiac abnormalities, while TTE reveals small septal communications more frequently.6 Echocardiography and CMR are both recommended for evaluating unoperated patients, informing on progressive disease and anatomies suitable of repair, as well as for monitoring operated patients, revealing surgical results and possible complications (figure 2).8 9
” data-icon-position data-hide-link-title=”0″>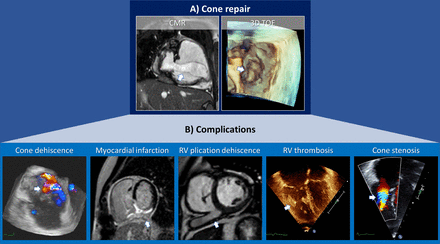

Cone repair assessed through multimodality imaging during diastole (A): vertical long axis view of cine-SSFP sequence from CMR imaging and en face view by 3D-TOE (ventricular perspective). White arrows indicate the cone-shaped valve. (B) Structural complications of cone repair, as highlighted by a white arrow: (I) en face neo valve view with dehiscence of the antero-septal region by colour flow Doppler 3D-TOE; (II) phase-sensitive inversion recovery reconstruction (PSIR) sequence of a mid-ventricular short-axis view, showing myocardial infarction in the territory supplied by the right coronary artery, as represented by transmural late gadolinium enhancement of the inferior wall, inferior septum and RV diaphragmatic wall; (III) cine-SSFP mid-ventricular short-axis view of right ventricular plication dehiscence; (V) colour flow Doppler 2D TTE of neo-subvalvular acceleration in a four-chamber view. CMR and TTE images are courtesy of Boston Children’s Hospital, Department of Cardiology. CMR, cardiovascular magnetic resonance; 3D-TOE, three-dimensional transesophageal echocardiography; RV, right ventricle; SSFP, steady-state free precession; TTE, transthoracic echocardiography.
TOE is the fundamental imaging technique in the operation theatre. Cardiac computed tomography may be considered instead of CMR in cases of contraindications or major artefacts in the magnetic field, as well as before planning redo surgeries or ‘valve-in-valve’ replacements.
Over time, various classifications of EA severity had been proposed (figure 3). In patients older than 10 years, the CMR-derived total right/left-volume index showed higher association with clinical decompensation compared with Celermajer index and fRV to left ventricle (LV) ratio (figure 3), but revealed no correlation with the occurrence of major cardiovascular events (MACEs).10–12 Imaging parameters predictive of adverse outcome in EA are reported in online supplemental tables 2 and 3.
” data-icon-position data-hide-link-title=”0″>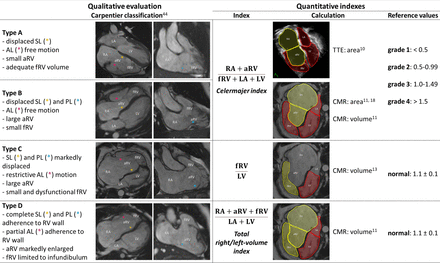

Classification of Ebstein’s anomaly from imaging. For the quantitative indexes, each term of the formula is highlighted in yellow when it is part of the numerator and in red when it is part of the denominator. AL, anterior leaflet; aRV, atrialised right ventricle; CMR, cardiac magnetic resonance; fRV, functional right ventricle; LA, left atrium; LV, left ventricle; PL, posterior leaflet; RA, right atrium; RV, right ventricle; SL, septal leaflet; TTE, transthoracic echocardiography.
Tricuspid valve
TV is usually incompetent in EA, being occasionally stenotic.1 Failure of delamination is responsible for a restrictive movement of the affected leaflet or its functional absence (online supplemental videos 1 and 2).1 6 AL may present fenestrations which contribute to tricuspid regurgitation (TR) as well as fibro-muscular attachments which cause RVOT obstruction.1 6
Comprehensive echocardiographic examination relies on multiple TV views, often obtained by tilting the TTE probe from conventional axis (figure 4) or adapting the TOE transducer to different orientations (online supplemental figure 1).
Supplemental material
” data-icon-position data-hide-link-title=”0″>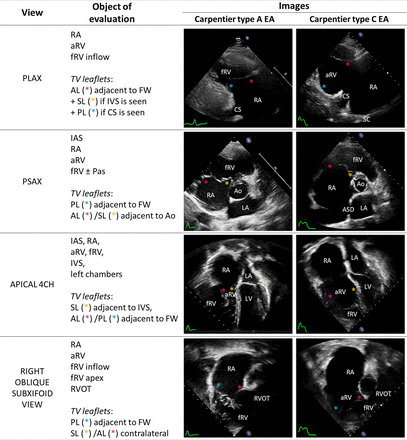

Main transthoracic echocardiographic views in EA. PLAX view was acquired with inferior tilt of the probe from conventional PLAX window. Right oblique subxifoid view was acquired with counterclockwise rotation of the transducer head from the four-chamber view. 4CH, four-chamber view; Ao, aorta; AL, anterior leaflet; aRV, atrialised right ventricle; ASD, atrial septal defect; CS, coronary sinus; EA, Ebstein’s anomaly; fRV, functional right ventricle; FW, free wall; IAS, interatrial septum; IVS, interventricular septum; LA, left atrium; LV, left ventricle; Pas, pulmonary arteries; PL, posterior leaflet; PLAX, parasternal long-axis view; PSAX, parasternal short-axis view; RA, right atrium; RVOT, right ventricular outflow tract; SL, septal leaflet; TV, tricuspid valve leaflets.
CMR allows a cross-reference between short-axis and long-axis views in the post-processing analysis, as illustrated in figure 1. Cine steady-state free precession (cine-SSFP) and gradient-echo sequences are used to evaluate TV leaflets’ morphology and motion. During a CMR examination, SL is detectable along the IVS. The other leaflets are visualised along the free wall: in a short-axis stack, PL is located next to the inferior wall and AL is adjacent to the lateral wall, while, in a para-axial stack from the diaphragm to the semilunar valves, PL and AL are detectable in bottom and top slices respectively (online supplemental figure 2). A vertical RV long-axis view explores both RV inflow and outflow, allowing the evaluation of PL delamination defects and possible RVOT obstruction; additionally, the degrees of the effective TV orifice rotation can be quantified in this view (figure 1D).4
An ‘en face’ reconstruction of TV is retrievable from multiple long-axis views using three-dimensional (3D) TTE (online supplemental video 3) and TOE (online supplemental figure 1).
Supplementary video
In mild disease, the TV offset from the anatomical annulus is modest and multimodal TR quantification is based on current recommendations. In case of significant TV orifice rotation from the anatomical annulus, regurgitant jets are generally better appreciated in long-axis rather than short-axis views. In these settings, TR echocardiographic evaluation is often qualitative, while CMR-based quantification is possible by subtracting total pulmonary antegrade flow from RV SV rather than using through-plane phase-contrast sequences orthogonal to the TV annulus.
TR is proportionally associated with decreased functional capacity in unoperated patients and its progression is accompanied by right-sided chambers volume overload and decreasing proportion of the left-sided chambers.11 13
Right atrium
RA is typically enlarged in EA and presents decreased either reservoir, conduit and booster pump function.14 Fibrosis is predominantly observed in the free wall and showed an association with atrial tachycardia onset.15 RA function is impaired in EA patients with heart failure (HF) and progressive disease.14 16
Right Ventricle
Although variability exists among different EA severity grades (figure 3), fRV is frequently enlarged and presents reduced systolic function when compared with normal subjects.14 17 Differently from echocardiographic techniques, CMR allows an unrestrictive view on right-sided heart chambers. In volume quantification, the para-axial cine-SSFP stack boundaries tracking showed a higher reproducibility than short-axis cine-SSFP stack (online supplemental figure 2).18 Late gadolinium enhancement is typically revealed in the site of TV leaflet delamination defects.15
Supplemental material
In regard to fRV function, the transverse rather than longitudinal component of contraction proved to be predominantly impaired in unoperated EA, even in the presence of preserved ejection fraction (EF).17
In general, data related to right-sided heart volumes mainly reflect cardiac decompensation and increased arrhythmic risk.11 12 14 Specifically, aRV volume proved to be a marker of exercise intolerance and QRS fractionation, which is a known predictor of arrhythmic events.13 19 20 Concomitantly, indexes of fRV systolic function revealed an association with MACEs, as detailed in online supplemental table 2.12 16
Left ventricle
The evidence that biventricular systolic impairment confers a ninefold increase in the risk of MACEs enlightens the impact of EA on left-sided heart.12
When compared with healthy subjects, LV shows reduced volumes, systolic and diastolic function.14 21 22 LV impairment in EA is multifactorial and principally triggered by: (1) interventricular dependency (aRV–LV and fRV–LV interaction); (2) reduced LV preload due to decreased forward RV SV; (3) shape anomalies including LV basal narrowing and apical dilation; (4) myocardial alterations, including features of cardiomyopathy (i.e. non-compaction, hypertrophic) and myocardial fibrosis (both focal and diffuse)2 21 23 24 (figures 5 and 6, online supplemental video 1 online supplemenal file 5).
” data-icon-position data-hide-link-title=”0″>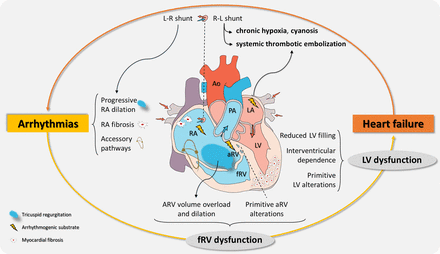

Clinical manifestations and pathophysiological substrates of Ebstein’s anomaly. The interplay between arrhythmias and heart failure is highlighted. RA dilation promotes the occurrence of intra-atrial re-entries and the presence of accessory pathways may be the substrate for atrioventricular re-entries; aRV is vulnerable to trigger ventricular arrhythmias. Fast-conduced arrhythmias may contribute to ventricular dysfunction and reduced filling time. Adverse myocardial remodelling may enhance the arrhythmic substrate. Increased RA pressures favour right-to-left shunt in the presence of atrial communications, thus worsening hypoxia. Systemic embolisation is favoured by occurrence of atrial fibrillation or paradoxical thrombosis. Ao, aorta; ARV, anatomical right ventricle; aRV, atrialised right ventricle; LA, left atrium; L-R, left to right; LV, left ventricle; PA, pulmonary artery; R-L, right to left; RA, right atrium.
” data-icon-position data-hide-link-title=”0″>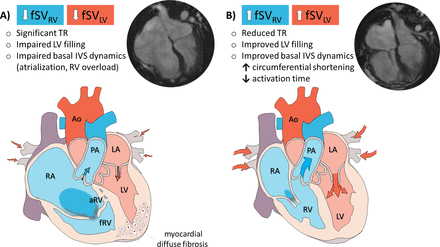

Structural and functional cardiac alterations in Ebstein’s anomaly (A) and changes after cone repair surgery (B). Also, a cine SSFP CMR sequence (horizontal long-axis view at end-diastole) is reported in the same patient before (A) and after cone repair (B). Ao, aorta; aRV, atrialised right ventricle; CMR, cardiovascular magnetic resonance; fRV, functional right ventricle; fSV, forward stroke volume; IVS, interventricular septum; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle; SSFP, steady-state free precession; TR, tricuspid regurgitation.
Clinical manifestations and outcome in unoperated patients
EA is a rare disease, counting 1–2.6 cases per 200 000 of live births (<1% of congenital heart disease).1 25 Clinical presentation after neonatal period has a more favourable outcome than cases diagnosed in earlier life.26 Subjects growing up to adulthood exhibit less severe anatomical abnormalities and, due to high right chambers compliance to volume overload, are usually asymptomatic for a long time.25 27 28 Mean age at diagnosis is around 14 years among children and 20–35 years in adults.25 26 New York Heart Association (NYHA) class I or II characterises the majority of adult patients at diagnosis.28 Arrhythmias are the most common clinical manifestation both in children and in adults, being a possible cause of NYHA class deterioration.25 28 Typical electrocardiographic and arrhythmic profiles of patients with EA are detailed in online supplemental material (sections C and D, respectively).
Right-to-left shunt may lead to chronic hypoxia and exertional cyanosis, while rest cyanosis is more common during infancy.26 Cerebrovascular embolic events, as assessed in around 8%–10% of cases, are attributable to either paradoxical embolization or atrial fibrillation occurrence.26 28 Endocarditis is rare and favoured by device implantation or surgery.26 29 Figure 5 illustrates distinctive EA symptoms and their physiological substrates.
Contemporary survival at 10 years from the diagnosis or at an age of 60 years old is assessed ≥80% among unoperated subjects.25 30 Causes of death are principally related to HF (40%) and arrythmias (20%).28 30 In a cohort of unoperated adults, sudden death (SD) had been reported in 23% of cases.28 Clinical and instrumental features associated with unfavourable clinical outcome are reported in online supplemental table 2.
- The Renal Warrior Project. Join Now
- Source: http://heart.bmj.com/cgi/content/short/110/4/235?rss=1
