Long-term heavy metal exposure, including Cd, Mn, Pb, Hg and other heavy metals, will not only lead to cytotoxicity, damage to body structures such as nervous system and hematopoietic system, but also seriously affect the function of the kidneys to excrete waste and toxins, and then lead to irreversible damage to human health25 . The concentration of metals in the blood can provide real-time information on metal exposure, distribution, and transportation within the body, while the concentration of metals in the urine reflects cumulative exposure and excretion. Both urine and blood samples were utilized for metal analysis, hoping to comprehensively assess the impact of metal exposure on human health. In this paper, urine Cd, urine Mn, blood Cd and blood Hg concentrations had wide distribution in overall population, whose 50th-75th quantile distribution range were more than twice of 25th-50th quantile distribution range, suggesting that there might be abnormal levels beyond the normal threshold.
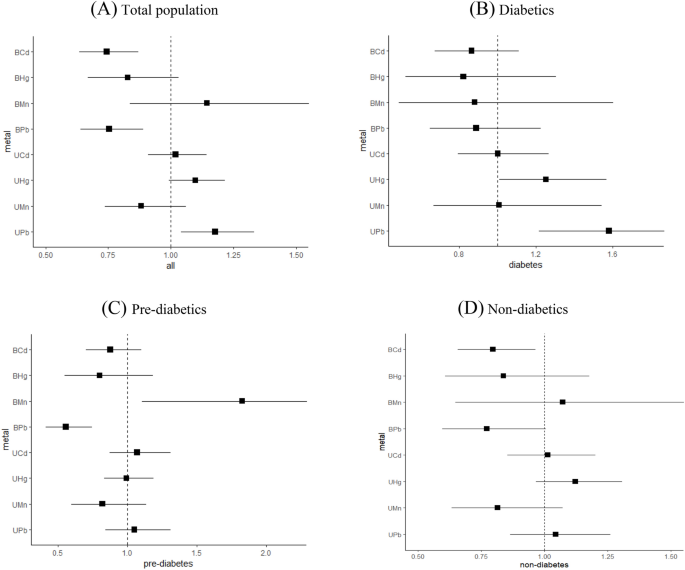
The cadmium absorbed by the human body does not play any physiological role26, even exposure to low doses of cadmium will show nephrotoxicity and carcinogenicity. However, we found no significant association between urine Cd and CKD in the total population or stratified analysis, monometallic effect or mixed metal effect analysis. It even showed a reduced risk of CKD in BKMR models, contrary to the expected results, but similar results have been shown in other studies27. It may be related to the hypothesis of reverse causality arising from cross-sectional studies. The level of urine cadmium decreases due to an increased risk of CKD, a decrease in eGFR, and the decreases of total filtration rate. In addition, Akerstrom et al.28 and Chaumont et al.29 suggested that protein excretion caused by Cd toxicity is unlikely at low levels of exposure, and factors that increase the variability of the biomarkers could either attenuate or overestimate an association.
Excessive lead intake will cause serious problems such as endothelial cell damage in kidneys, renal fibrosis, and glomerulosclerosis. We found that urine Pb increased the risk of CKD in overall study subjects, people over 60 years old and diabetics, while blood Pb showed an opposite effect in overall populations, people over 60 years old and pre-diabetics by monometallic Logistic regression analysis. Most current studies suggested an association between urine lead and renal insufficiency30,31, but the association between blood lead and renal impairment is still controversial. Considering the similarity between lead and cadmium, it may be due to the effects of hyperfiltration by decreased renal function.
We found a positive association between blood Mn and the CKD in pre-diabetics. Manganese plays an important role in glucose, lipid, and protein metabolism, and may be associated with diabetes and CKD through substance metabolism disorders6. However, some studies have proposed a negative association or no association between plasma manganese and CKD32,33. The measurement of Blood Mn is considered as an indicator for current Mn exposure. Most biomarker studies related to occupational inhalation of Mn have primarily focused on plasma or serum Mn. However, several studies have indicated a weak correlation between plasma Mn concentrations and external exposure levels34,35. Therefore, the specific association between blood manganese and CKD needs to be further studied.
Most of the metabolic mercury will be excreted with urine, but excess mercury will also cause damage to kidney. In this paper, urine Hg increased the risk of CKD in people aged 40–59 years old and people with T2DM. A review suggested that individuals with decreased kidney function due to CKD or other causes like diabetes may be more likely to promote the progression of kidney damage after exposure to mercury, which may partly explain the positive association between urine mercury and CKD in people with T2DM36.
Simple generalized linear regression models, including multivariate Logistic regression and linear regression, are commonly used to assess the impact of environmental factors on human health37, but the results may be distorted due to the inability to discern potential antagonism and synergy between exposures. WQS regression model combines exposure percentile weighted scores with Logistic regression to assess changes in metal mixtures and the risk of CKD38. Based on bootstrap sampling experience to evaluate the overall effect and determine the weight of each exposure, WQS regression can effectively avoid the interference of metal collinearity, reveal complex exposure risks closer to real life, and have higher sensitivity, specificity and accuracy than univariate analysis in identifying important factors. In this paper, WQS regression was used to fit the association between metal mixed exposure and the risk of CKD. The results showed that urinary metal mixed exposure increased the risk of CKD, and this association was more pronounced in stratified analysis of gender, age, and blood glucose status. Blood metal mixture exposure also increased the risk of CKD after stratification by blood glucose status, and the effect was more significant than urinary metal mixture exposure, further confirming the association between metal exposure and the risk of CKD.
The results of metal weight distribution in some WQS regression models were consistent with the results of single-metal analysis. We found that WQS regression models determined different metal weights after stratified analysis because the WQS weighted index represents an average effect39, thus it is possible to overestimate or underestimate the overall effect of mixed metal exposure and the weight of each metal in a particular population.
Most previous studies have used statistical methods to explore the association between metal exposure and renal impairment, which cannot address the complex nonlinear effect and the potential interactions between mixed exposures. Bobb et al.24 proposed Bayesian Kernel Machine Regression (BKMR) methods, which can be used to estimate the nonlinear, non-additive exposure response relationship between mixed metal exposure and CKD, explore the exposure effects of individual metal when other metals are at a fixed level, and identify potential interaction between metals23. Compared with the traditional regression model, BKMR model can better deal with multicollinearity problem and unknown overall effect and be closer to the real exposure. Compared to WQS regression model, BKMR can account for possible interaction between exposed metals and avoid false positive results due to ignoring interaction40. Using BKMR model, Luo and Hendryx41 concluded that exposure to a mixture of four metals (cobalt, chromium, mercury, lead) was associated with decreased kidney function in American adults aged 40 years and older; Zhou et al.27 found a linear dose–response association between metal mixtures and CKD in elderly diabetics in a Chinese community. We also used BKMR model and observed an obvious association effect between blood metal mixed exposures and CKD, which was more easily observed in stratified analysis. It can also explain to some extent the current research and analysis, which used blood levels to assess the association between metal exposure and kidney function in order to minimize the possible reverse causal relationship. When other metals were fixed at specific percentiles (25th, 50th, 75th percentiles), the effect of individual metal increasing from 25th percentiles to 75th percentiles on CKD were calculated. Urine mercury and blood cadmium were positively associated with the risk of CKD in the overall study subjects and in patients with T2DM, which was different from the results of univariate analysis because of the random distribution of other metals. BKMR method is also somewhat restrictive due to its core algorithm. We can only infer the exposure–response function when other metals are fixed at a certain level. The effect caused by both high-level and low-level metal exposure cannot be estimated.
In addition, multiple heavy metals often coexist in humans, and mixed metal exposure has been proved to have additive and/or synergistic effects on renal function42. It may cause renal impairment through common mechanisms such as oxidative stress. The effect of mixed metal on renal function is depending on the nature, dose, route, and duration of exposure, and may be stronger in patients with T2DM due to the additive effect. In overall population, there were interactions between urine Pb and other three urine metals, blood Cd and blood Mn, blood Cd and blood Pb, blood Mn and blood Pb. In people with T2DM, except for the lack of interaction between blood Cd and blood Pb, the other 5 interactions still existed, and the interaction between urine Cd and urine Mn, urine Cd and urine Hg, blood Hg and blood Mn, blood Hg and blood Pb were added. Chen et al.43 found that co-exposure to lead and cadmium may worsen cadmium or lead induced tubular dysfunction alone. Another review suggested that combined exposure to lead and mercury increased ROS accumulation in cells and caused oxidative damage compared with a single exposure44. Yang et al.45 used BKMR model to observe the combined effect of blood manganese with blood cadmium and blood lead on eGFR. Several studies have shown that interaction between metal exposures plays an important role in health effect, and future relevant prospective and large-sample studies should not overlook the interaction between exposures.
This paper also has certain limitations. First of all, it is a cross-sectional study with a small sample size, which cannot demonstrate causality and it is possible to observe reverse causation. Secondly, only a single time measurement of urine and whole blood concentrations of Cd, Mn, Pb and Hg were selected as exposure factors. Fewer types of metals were restrictive in reflecting the real exposure of human body. It can only reflect the measured metal content of recent human exposure with a short half-life, and cannot fully explain the accumulation effect of long-term metal exposure. It is also crucial to consider the impact of urine dilution (measured by urinary creatinine or specific gravity) when evaluating exposure based on urine samples. More nephrotoxic metals and plasma metal concentrations should be considered, and attention should be given to adjusting the indicators in order to explore the association between mixed metal exposure and renal function impairment, and provide a theoretical basis for selecting appropriate indicators. In addition, we evaluated the renal function based on the serum creatinine concentration measured at a single time, and calculated eGFR by the CKD-EPI formula, which cannot fully reflect the true status of renal function of population. It is recommended to add other renal function damage markers such as urine albumin, cystatin C and other indicators to comprehensively reflect the kidney function status. At last, the analysis was conducted based on unweighted samples, which undeniably causes some sample bias and estimation error. We had no covariates information on diet and medication so we cannot exclude confounding bias due to other potentially influencing factors.
- The Renal Warrior Project. Join Now
- Source: https://www.nature.com/articles/s41598-024-63858-3
