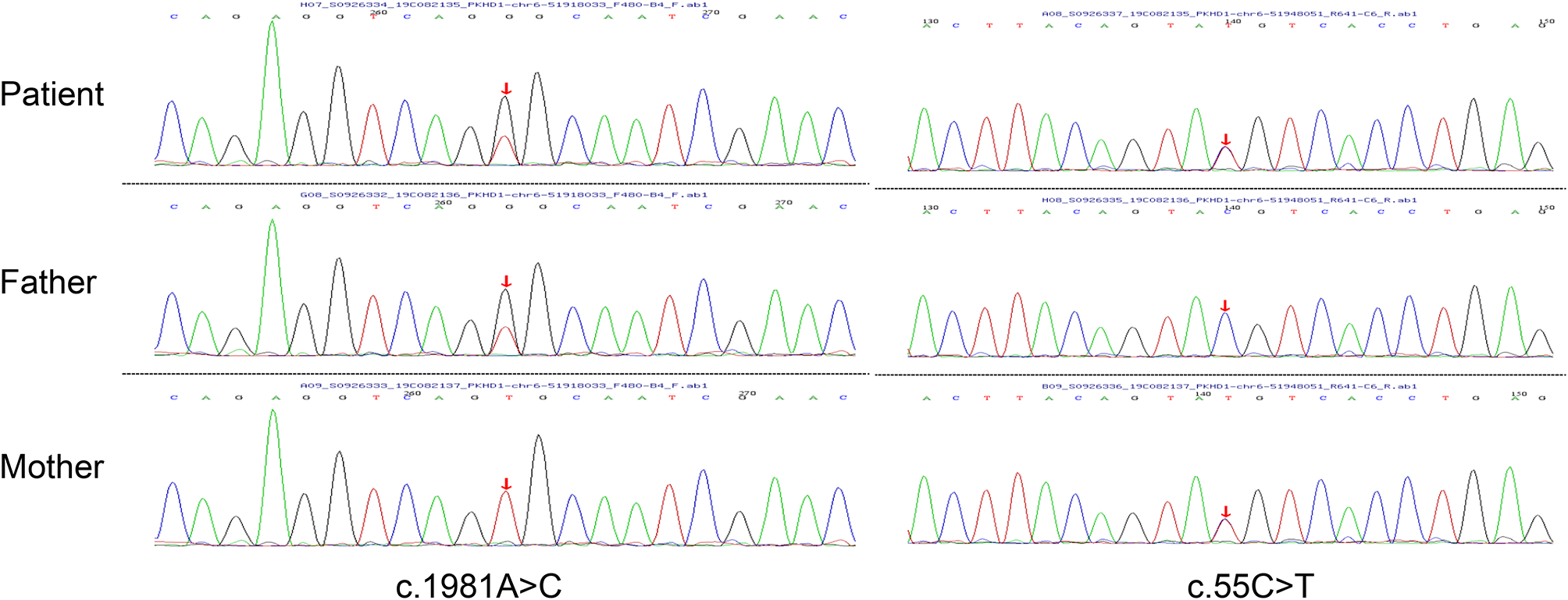
Herein, we reported a case of ARPKD accompanied with NVM. The girl was found to have ARPKD at five months old and have normal kidney functions during four years’ follow-up. She was accidently found to have NVM and chronic heart failure.To our knowledge, no cases of ARPKD accompanied with NVM have been previously reported.
Cardiac damage has rarely been reported in patients with ARPKD. Chinali et al. conducted ultrasound examinations on 27 children with ARPKD, of which 17/27 (63%) had a creatinine clearance < 90 mL/min, 3 patients had stage IV chronic kidney disease, 5 patients were on peritoneal dialysis and 19/27 (74%) had hypertension. It was found that compared to 88 normal controls, patients with ARPKD exhibited a higher left ventricular mass index(38.7 ± 12 vs.28.9 ± 4.3 g/[m2.16 + 0.09]) and subclinical systolic dysfunction, with a significant decrease in midwall FS and circumferential strain despite normal EF values.Children with ARPKD show significantly impaired cardiac phenotype [9].
ARPKD is a type of renal ciliopathy in which primary cilia play a central role in the pathogenesis [10]. The cilia also play an essential role in regulating the signal cascade required for forming left and right asymmetries in the early stages of cardiac development [11]. Li et al. [12] conducted ultrasound scans on 87,355 chemically mutated C57BL/6J fetal mice. A wide spectrum of congenital heart disease (CHD) phenotypes were recovered, including single pulmonary artery, ventricular septal defect, aortic atresia with ventricular septal defect, heterotaxy, ventricular septal defect, interrupted aortic and so on. 91 recessive CHD mutations in 61 genes were identified by whole exome sequencing, including 34 ciliated genes. Sixteen genes were involved in ciliated cell signaling and ten genes were involved in vesicular transport, including polycystic kidney disease (PKD) 1, PKD2, coiled-coil domain containing 151 and dynein assembly factor with WDR repeat domains 1. Confocal imaging of E12.5 Cc2d2a-mutant mouse showed no cilia in the atrioventrcular cushion. Fewer and shorter cilia were observed in other mutant embryo tissue. This suggests that cilia and cilia-induced signal transduction play an essential role in the pathogenesis of CHD.
PKD1/2, involved in CHD, are the pathogenic genes for autosomal dominant polycystic kidney disease (ADPKD) [10]. As another kind of PKD, ADPKD was more related to cardiovascular diseases. Of 667 ADPKD patients, 5.8% had idiopathic dilated cardiomyopathy, 2.5% had hypertrophic obstructive cardiomyopathy, and 0.3% had left ventricular noncompaction [13]. There are relationships between the genes of ARPKD and ADPKD. The PKHD1 gene product FPC co-localizes with the PKD2 gene product polycystin-2 (PC2) – an intracellular calcium channel expressed in renal epithelial and myocardial cells. After PKHD1 mutation, a decrease in FPC protein can reduce PC2 and PC2 channel activity [14]. PC2 are expressed in many tissues including vascular smoothmuscle cells and cardiomyocytes. PKD2 mutations in ADPKD patients could alter the expression of PC2, thus directly impairing intracellular calcium circulation and affecting the function of vascular smooth muscle and cardiomyocytes [15]. Meanwhile, enlargement of cysts in the kidney could lead to renal arteriolar attenuation and ischemia, thus activating renin-angiotensin-aldosterone system. ADPKD could also cause the increase of fibroblast growth factor, then contributing to hypertension and left ventricular hypertrophy [16]. Thus, ADPKD patients may exhibit symptoms such as cardiomyopathy and NVM [13]. The coexistence of ARPKD and NVM may be caused by the alterations of PC1 and PC2 channel activity regulated by FPC. The continuity of NVM with other ciliopathies may indicate that NVM is also a ciliopathy.
In conclusion, the association between ARPKD and cardiac damage remains uncertain. It is unsure if the coexistence of NVM and ARPKD is a coincidence or they are different manifestations of ciliary dysfunction in the heart and kidneys.
- The Renal Warrior Project. Join Now
- Source: https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-024-03642-7
